Answered step by step
Verified Expert Solution
Question
1 Approved Answer
label them as spontaneous or nonspontaneous. (a) 2SO_(2)(g)+O_(2)(g)2SO_(3)(g);Delta H^(0)=-197.8kJ,Delta Sdeg =-188.0(J)/(K) Delta G^(0)=,kJ, and the reaction is-Select-- hat(v). (b) 2C_(6)H_(6)(l)+15O_(2)(g)12CO_(2)(g)+6H_(2)O(l);Delta H^(0)=-6535.0kJ,Delta S^(0)=-439.2(J)/(K)
label them as spontaneous or nonspontaneous.\ (a)
2SO_(2)(g)+O_(2)(g)2SO_(3)(g);\\\\Delta H^(0)=-197.8kJ,\\\\Delta S\\\\deg =-188.0(J)/(K)\
\\\\Delta G^(0)=,kJ, and the reaction is-Select-- hat(v).\ (b)
2C_(6)H_(6)(l)+15O_(2)(g)12CO_(2)(g)+6H_(2)O(l);\\\\Delta H^(0)=-6535.0kJ,\\\\Delta S^(0)=-439.2(J)/(K)\
\\\\Delta G^(0)=,kJ, and the reaction is - Select- hat(v). 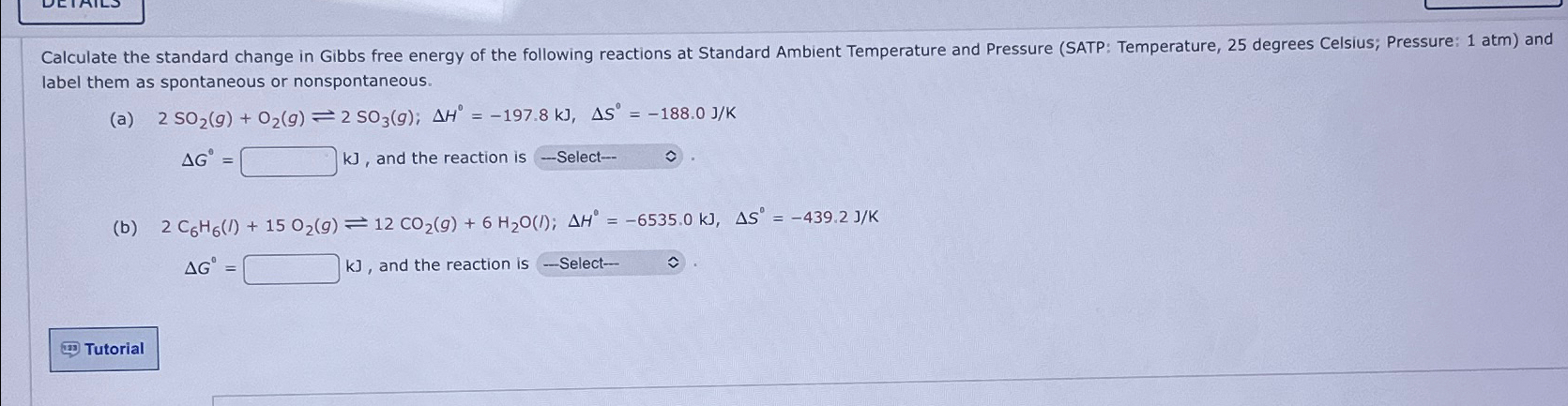
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


