Question
Lake Case Study Lab Water Sampling NEED THE ANSWER FOR PART 2, I added part 1 too but I only NEED part TWO !!! (its
Lake Case Study Lab Water Sampling
NEED THE ANSWER FOR PART 2, I added part 1 too but I only NEED part TWO !!! (its at the bottom )
Lake Study, Part 1
Analyzing data and developing a hypothesis. This part of the case study is devoted to analyzing the data given to form a hypothesis about what is causing the breathing problems in the fish. Because of the suspicion that something in the water is leading to the breathing difficulties that the fish are experiencing, the fish hatchery supervisor ordered samples of the lake water be taken and analyzed. She mentions the following potential causes of the problem.
1. Dissolved Oxygen: If the levels of dissolved oxygen in the lake were too low, the fish would have trouble breathing and may die.
2. Metals: The presence of dissolved metals in the lake may cause problems if the metal concentrations are high enough.
3. Pesticides: High levels of pesticides have been shown to be harmful to fish.
The background information provided will help you identify the environmental cause of the mortality of the hatchery trout.
Analytical laboratory procedures: Ten different water samples from various areas around the lake were taken and sent to an analytical laboratory. Three types of analysis were performed. For statistical purposes, the more samples you take the more reliable the resulting analysis becomes. The greater the sample size, the more data there is. However, in a research setting, it is not economically feasible to collect and run tests on a very large number of samples. A good rule of thumb to follow is to test in triplicate. If you can test more than that, your data is just that much more reliable.
Another consideration that needs to be taken in to account is where the samples were taken. Do you want all of your test samples to be taken in the same vicinity or do you want to spread them out? For thorough testing, you would want a wide range of sample areas (take some near the shore, near the dock, in the middle of the lake, etc...).
* Dissolved Oxygen: You can titrate for dissolved oxygen. The results are reported in ppm oxygen. 1 ppm = 0.001 g/liter
* Metals: You can analyze for four different metals: calcium, lead, mercury, and iron. The method used for metal analysis is atomic absorption (AA) spectroscopy, a modern analytical method using an instrument. In an atomic absorption spectrophotometer a liquid sample is introduced into a flame. When the sample enters the flame, the water immediately evaporates and the metals in the sample vaporize. This produces a high concentration of metallic atoms in the flame. A beam of light is directed through the flame and the metallic atoms absorb some of this light. The amount of light that is absorbed tells you the concentration of metals originally dissolved in the liquid sample. A summary of the method follows:
1. Unknown sample is introduced into flame.
2. Flame vaporizes metals in sample producing metallic atoms.
3. Metal atoms absorb part of a light beam shining through flame.
4. Light absorbed is measured to determine how much metal was in sample.
The AA results are reported in parts per million, ppm (1 ppm = 0.001 g/liter), except for mercury, which is reported in parts per billion, ppb (1 ppb = 0.000001 g/liter).
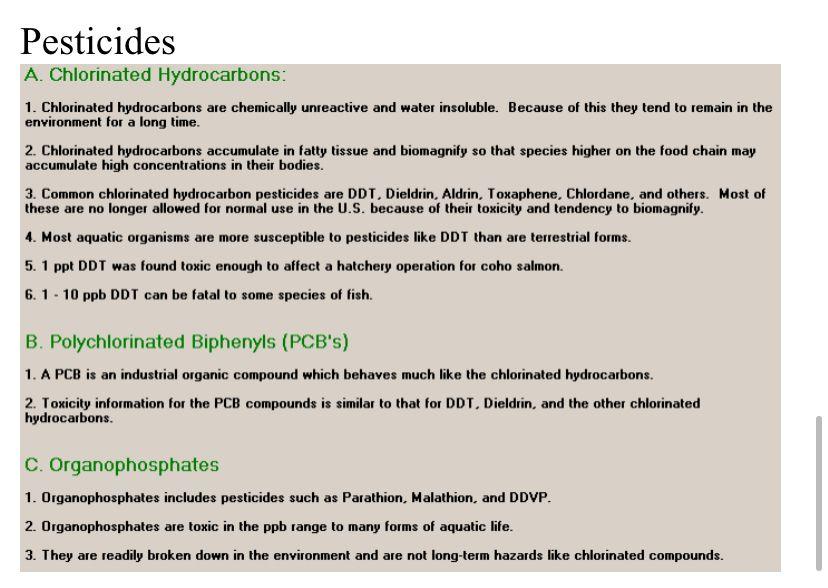
Pesticides: An extremely important analytical method called Gas Liquid Chromatography (GLC) is used to determine the pesticide levels in the water. Three common environmental contaminants DDT, Dieldrin, and PCB's are determined. In GLC, a liquid sample containing a mixture of pesticides is injected into the instrument with a syringe. Inside the instrument the pesticides move through a column at different rates, which separates them from each other so that they exit the column at different times. When the pesticides come out of the column, they pass through a detector, which sends a signal to a recorder. The signal is registered as a peak on a piece of moving chart paper. The position of the peaks tells you what the substances are and size of the peaks tells you how much of each is in the mixture.
Pesticide levels are reported in parts per trillion, ppt.
1 ppt = 0.000000001 g/liter
Results
This week's analyses of the lake water show results in the following ranges:
Temperature 1720 C
Calcium 2026 ppm
Lead 0.60.72 ppm
Mercury 2-4 ppb
Iron 1.21.8 ppm
Pesticides low (fractional ppt range)
D.O. more than adequate for aquatic life
Assignment PART 1 : Are the species the same as the hatchery trout? For example is the data involving dogfish, a type of shark found in salt water, pertinent to fresh water hatchery trout?

 PART 2
PART 2
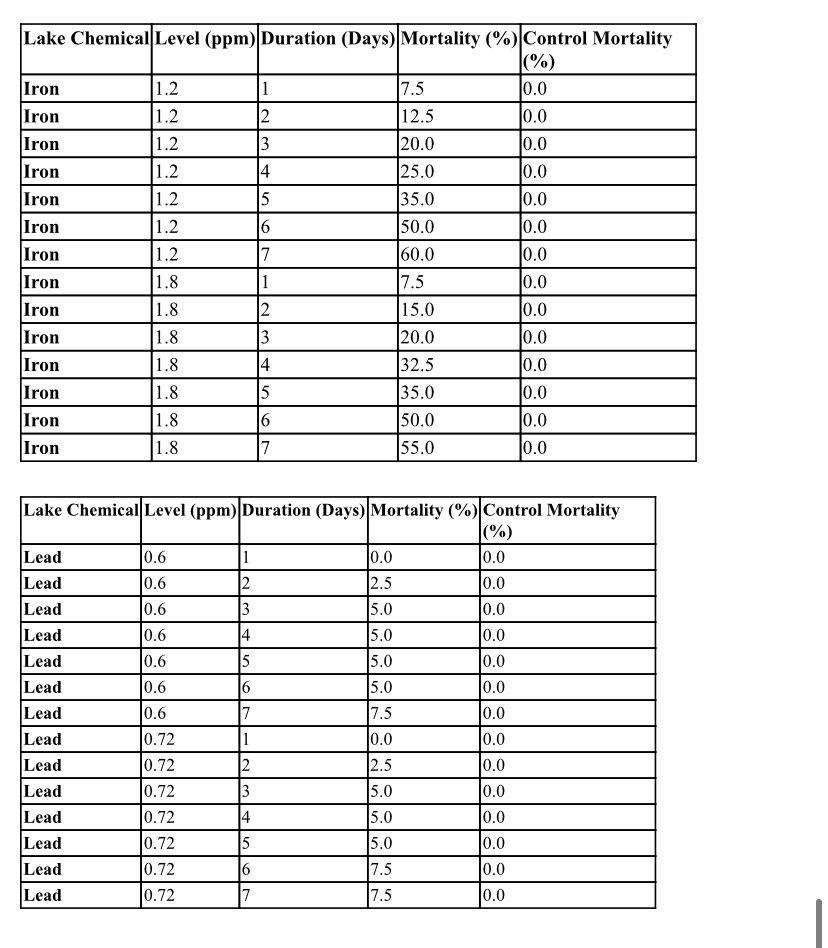
After some initial experiments the cause of the breathing problems has been narrowed to the presence of either iron or lead in the water of the lake. The results of these experiments are not clear-cut however, so some additional controlled experiments in which the conditions are known must be done to try to establish the culprit.
In this part of the experiment, the laboratory was used again. This time fish tanks were available to observe the fish under controlled conditions to see how they behave (in
particular, if they die). The number of fish who die in a tank under a given set of conditions can be compared to a tank that has all the same conditions except for the variable being
tested.
As an example, if a researcher wanted to test the effect of a 100ppm concentration of Ca on fish at 20 C, they could look at two tanks: a control tank containing plain water at 20C and another tank containing water with 100ppm Ca at 20C. If significantly more fish die in the 100ppm Ca tank than in the control tank, then we would suspect that 100ppm Ca is toxic to fish. Controlled tests on the fish under appropriate conditions were performed to decide whether iron or lead was causing the breathing problems. Below is the data that was obtained.
ANSWER FOR PART 1 :
The case study is about fishes living in a lake which are dying due a suspicious reason. The unknown reason can include any of the 3 affects which are:
1)Low Dissolved Oxygen: If the levels of dissolved oxygen in the lake were too low, the fish would have trouble breathing and may die.
2)High concentration of Metals: The presence of dissolved metals in the lake may cause problems if the metal concentrations are high enough.
3)High concentration of Pesticides: High levels of pesticides have been shown to be harmful to fish.
Now we have selected the sample of the water from 10 different areas of the lake so that we can get the actual reason of this cause . By taking various sample our report can have a better accuracy and precision of results. Calculating the amount of metals and pesticides in water from differents regions of lake can be helpful in knowing thw cause of the problems that from where these hazardous elements are being added and we can eliminate them from being added to the water. So we took samples from different parts and tested the presence of various metals in water and upto how much concentration. After getting the results from the lab we can conclude that the Dissolved Oxygen is not a problem as it is sufficient to support forms in water. Next we came to metals concentration , with the data given we can comment that the amount of metals like Iron and Lead is much higher than the normal concentration in water and thus this could be a possible threat to the aquatic life forms. The results of lab reports also showing vey less percentage of the pesticides and thus only 1 potential threat is there which is higher concentration of metals present in the lake water. Looking at the various i can comment that we the concentration of iron and lead is increasing for every day by a few percentage and also prolonged exposure of these metals for a longer period of time which are affecting not only the mature fishes but also the egg hatchings. Due to the exposure of these metals we are facing huge increase in the mortality rate of the fishes and other aquatic lifeforms . So its high time that we need to control all this before that lake turns into a pool of heavy chemical metals. We need to look at all the potentials threats and treat them properly , we can introduce biological metals eating bacterias ( Pseudomonas aeruginosa) which can level the concentration of metals in water . We need to take quick steps in order to secure the life of the rest of the aquatic lifeforms present there
PART 2 ANSWER THIS QUESTION PLEASE CHEGG PERSON Ill RATE THIS AFTERWARDS AND COMMENT POSSITIVE IF NEEDED SAME
PART 2 EXERCISE DESCRIBE THE PROCEDURE USED IN THE LAKE STUDY EXPERIMENT INVOLVING IRON AND LEAD. INCLUDE IN THE DESCRIPTION THE IRON AND THE LEAD CONCENTRATIONS, NUMBER OF TANKS EMPLOYED, THE TIME PERIOD OF THE EXPERIMENR AND ANY CONTROLS AND REPORT THE RESULTS OBTAINED IN THE LAKE STUDY EXPERIMEN AS DESCRIVEN IN THE DATA TABLE ABOVE
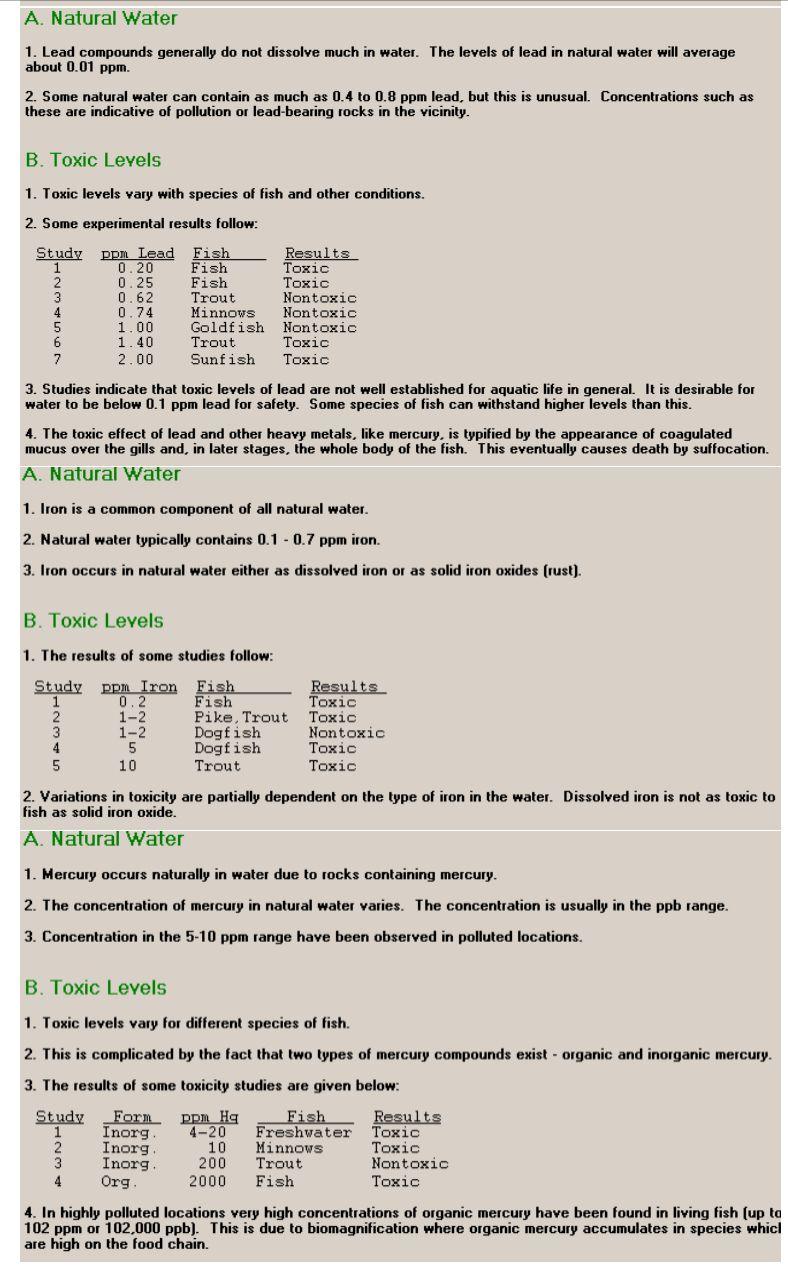
Dissolved Oxygen Concentrations: Chemicals in the environment often are present in very small concentrations. These concentrations are usually expressed in the following units: ppm = parts per million (roughly 4 drops in a 50 gallon barrel) ppb - parts per billion (roughly 1 drop in a railroad tank car) ppt - parts per trillion (roughly 1 drop in 1000 tank cars) Ppq - parts per quadrillion (roughly 1 drop in a 6-mile high column of water above a football field) 1 ppm - 1 mg/kg 1 ppb = 1 g/kg 1 ppt = 1 ng/kg 1 ppq = 1 pg/kg 1 ppm = 1000 ppb = 1,000,000 ppt = 1,000,000,000 ppg Dissolved Oxygen A. Natural Conditions 1. Natural water typically contains 8 - 11 ppm dissolved oxygen. 2. The amount of dissolved oxygen varies with temperature. 3. Low oxygen concentrations are an indicator of some types of pollution. B. Toxic Levels 1. The lack of oxygen can cause death or breeding problems in fish. 2. Some species are more resistant to low dissolved oxygen than others. 3. Generally, levels of dissolved oxygen below 5.0 ppm are harmful to fish and other aquatic life. Heavy Metals- Calcium, Lead, Iron, Mercury A. Natural Water 1. Calcium is a common constituent of natural water because of contact with limestone and other calcium-rich natural rocks. 2. Calcium is the main component in hard water. Normal water contains from 15 to 52 ppm calcium. Hard water can contain 100 ppm or more. B. Toxic Levels 1. Calcium is not toxic at most levels and generally is not harmful to aquatic life. 2. Sometimes the presence of high levels of calcium can mitigate the effects of other pollutants. For instance, 50 ppm calcium has been shown to cancel the toxic effects of 2 ppm zinc and 0.7 ppm lead on some species of fish. A. Natural Water 1. Lead compounds generally do not dissolve much in water. The levels of lead in natural water will average about 0.01 ppm. 2. Some natural water can contain as much as 0.4 to 0.8 ppm lead, but this is unusual. Concentrations such as these are indicative of pollution or lead-bearing rocks in the vicinity. B. Toxic Levels 1. Toxic levels vary with species of fish and other conditions. 2. Some experimental results follow: Study PPM Lead Fish Results 0.20 Fish Toxic 2 0.25 Fish Toxic 3 0.62 Trout Nontoxic 0.74 Minnows Nontoxic 5 1.00 Goldfish Nontoxic 6 1.40 Trout Toxic 7 2.00 Sunfish Toxic 3. Studies indicate that toxic levels of lead are not well established for aquatic life in general. It is desirable for water to be below 0.1 ppm lead for safety. Some species of fish can withstand higher levels than this. 4. The toxic effect of lead and other heavy metals, like mercury, is typified by the appearance of coagulated mucus over the gills and, in later stages, the whole body of the fish. This eventually causes death by suffocation. A. Natural Water 1. Iron is a common component of all natural water. 2. Natural water typically contains 0.1 -0.7 ppm iron. 3. Iron occurs in natural water either as dissolved iron or as solid iron oxides (rust). B. Toxic Levels 1. The results of some studies follow: Study 2 DOM Iron Fish 0.2 Fish 1-2 Pike, Trout 1-2 Dogfish 5 Dogfish 10 Trout Results Toxic Toxic Nontoxic Toxic Toxic 4 2. Variations in toxicity are partially dependent on the type of iron in the water. Dissolved iron is not as toxic to fish as solid iron oxide. A Natural Water ury occurs naturally water due rocks ning mercury. 2. The concentration of mercury in natural water varies. The concentration is usually in the ppb range. 3. Concentration in the 5-10 ppm range have been observed in polluted locations. B. Toxic Levels 1. Toxic levels vary for different species of fish. 2. This is complicated by the fact that two types of mercury compounds exist - organic and inorganic mercury. 3. The results of some toxicity studies are given below: Study 2 For Inorg Inorg Inorg Org ppm Hg 4-20 10 200 2000 Fish Freshwater Minnows Trout Fish Results Toxic Toxic Nontoxic Toxic 4 4. In highly polluted locations very high concentrations of organic mercury have been found in living fish (up ta 102 ppm or 102,000 ppb). This is due to biomagnification where organic mercury accumulates in species whicl are high on the food chain. Pesticides A. Chlorinated Hydrocarbons: 1. Chlorinated hydrocarbons are chemically unreactive and water insoluble. Because of this they tend to remain in the environment for a long time. 2. Chlorinated hydrocarbons accumulate in fatty tissue and biomagnify so that species higher on the food chain may accumulate high concentrations in their bodies. 3. Common chlorinated hydrocarbon pesticides are DDT, Dieldrin, Aldrin, Toxaphene, Chlordane, and others. Most of these are no longer allowed for normal use in the U.S. because of their toxicity and tendency to biomagnify. 4. Most aquatic organisms are more susceptible to pesticides like DDT than are terrestrial forms. 5.1 ppt DDT was found toxic enough to affect a hatchery operation for coho salmon. 6.1.10 ppb DDT can be fatal to some species of fish. B. Polychlorinated Biphenyls (PCB's) 1. A PCB is an industrial organic compound which behaves much like the chlorinated hydrocarbons. 2. Toxicity information for the PCB compounds is similar to that for DDT, Dieldrin, and the other chlorinated hydrocarbons. C. Organophosphates 1. Organophosphates includes pesticides such as Parathion, Malathion, and DDVP. 2. Organophosphates are toxic in the ppb range to many forms of aquatic life. 3. They are readily broken down in the environment and are not long-term hazards like chlorinated compounds. 1.2 Lake Chemical Level (ppm) Duration (Days) Mortality (%) Control Mortality (%) Iron 1.2 1 7.5 0.0 Iron 1.2 2 12.5 0.0 Iron 3 20.0 0.0 Iron 1.2 4 25.0 10.0 Iron 1.2 5 135.0 0.0 Iron 1.2 16 50.0 10.0 Iron 1.2 7 160.0 10.0 Iron 1 17.5 10.0 Iron 1.8 2 15.0 10.0 Iron 1.8 3 20.0 10.0 Iron 1.8 4 32.5 0.0 Iron 1.8 15 35.0 10.0 Iron 1.8 16 50.0 10.0 Iron 1.8 7 55.0 0.0 NIN 1.8 AIN Lake Chemical Level (ppm) Duration (Days) Mortality (%) Control Mortality (%) Lead 0.6 1 0.0 0.0 Lead 0.6 2 2.5 10.0 Lead 0.6 3 5.0 0.0 Lead 0.6 14 5.0 0.0 Lead 0.6 5 5.0 0.0 Lead 10.6 6 5.0 0.0 Lead 0.6 7 7.5 0.0 Lead 0.72 1 10.0 0.0 Lead 10.72 2 2.5 0.0 Lead 0.72 3 5.0 0.0 Lead 0.72 4 5.0 0.0 Lead 0.72 5 5.0 10.0 Lead 0.72 16 7.5 0.0 Lead 0.72 7.5 0.0 7
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


