Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Las soluciones de metanol y etanol son b sicamente ideales. a ) Calcule los equilibrios vapor - liquido para este sistema a 1 y 5
Las soluciones de metanol y etanol son bsicamente ideales.
a Calcule los equilibrios vaporliquido para este sistema a y atm abs de presin y graficar
los diagramas y a cada presin
b Para cada presin calcule las volatilidades relativas y determine un valor promedio.
c Utilizando la ecuacin con las volatilidades promedio; compare los valores de en
cada valor de obtenido de esta forma, con los valores calculados directamente a partir
de las presiones de vapor.
Ecucacin
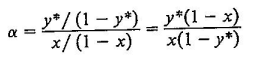
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


