Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Learning Goal: To calculate average and relative reaction rates. You can measure the rate of a reaction, just like you can measure the speed a
Learning Goal:
To calculate average and relative reaction rates.
You can measure the rate of a reaction, just like
you can measure the speed a jogger runs. While a
jogger would be reported to run a specific number
of miles in an hour, mileshour a reaction is
reported to form product or consume reagent in
molar concentration per second,
Reaction rate can be defined either as the increase
in the concentration of a product per unit time or as
the decrease of the concentration of a reactant per
unit time. By definition, reaction rate is a positive
quantity.
In the reaction for example, is being
produced twice as fast as is consumed and thus
rate rate
Each rate can be expressed as the change in
concentration over the change in time, :
Rate of decomposition of
Previous Answers
Correct
For every two moles of that disappear, only one mole of is formed. The rate of
decomposition of is twice the rate of formation of
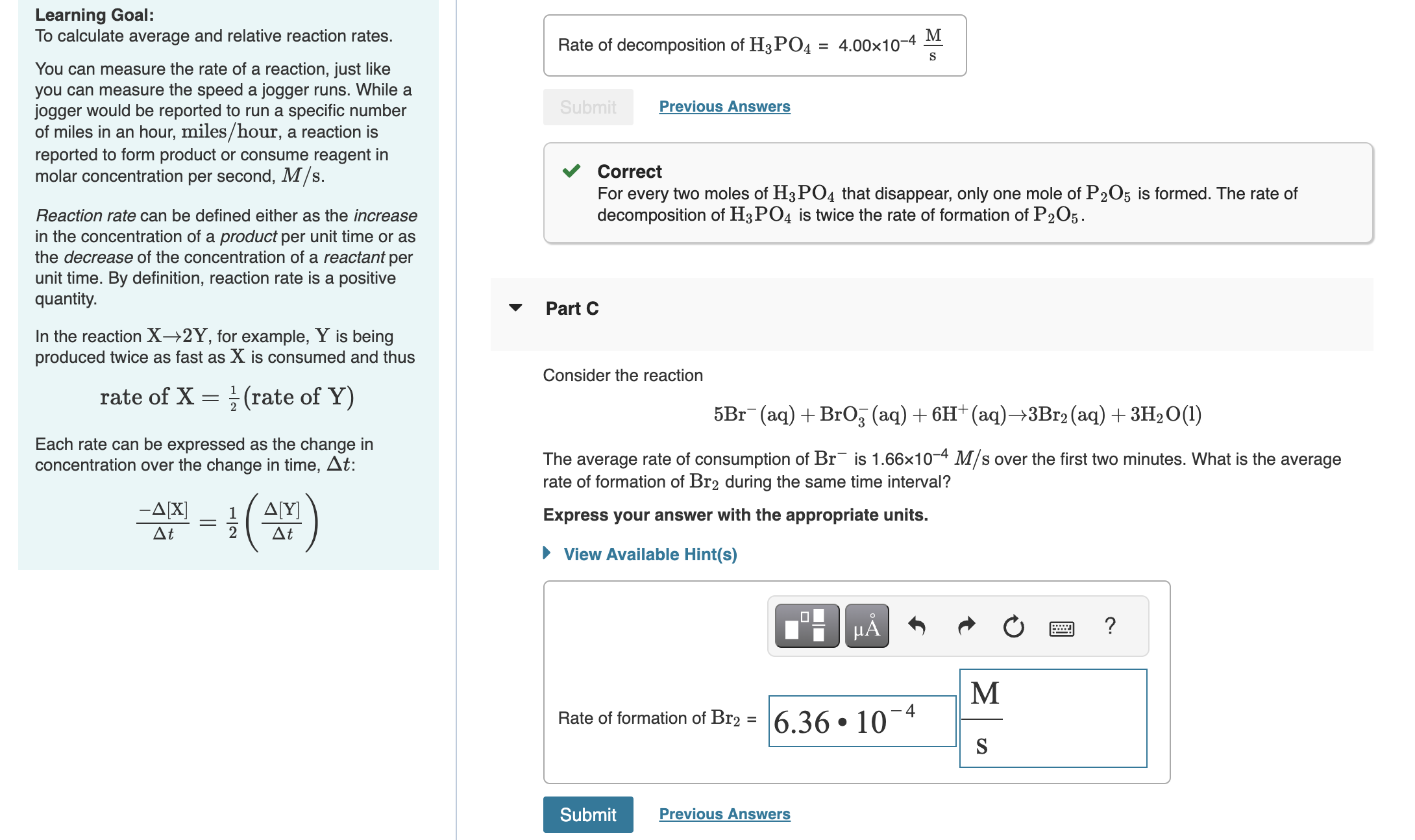
Part C
Consider the reaction
The average rate of consumption of is over the first two minutes. What is the average
rate of formation of during the same time interval?
Express your answer with the appropriate units.
View Available Hints
Rate of formation of
M
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


