Answered step by step
Verified Expert Solution
Question
1 Approved Answer
LHV = HHV -mwaterHevaporation of water mwater = water contect of fuel + water content of air + water generated by the reaction + H2
LHV = HHV -mwaterHevaporation of water
mwater = water contect of fuel + water content of air + water generated by the reaction
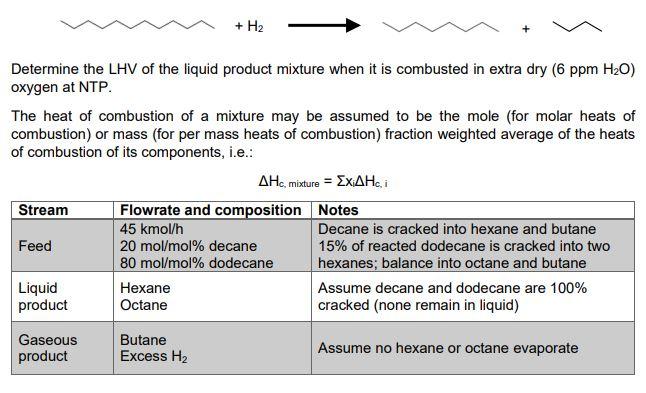
+ H2 Determine the LHV of the liquid product mixture when it is combusted in extra dry (6 ppm H20) oxygen at NTP. The heat of combustion of a mixture may be assumed to be the mole (for molar heats of combustion) or mass (for per mass heats of combustion) fraction weighted average of the heats of combustion of its components, i.e.: AHC, mixture = ExAHI Stream Flowrate and composition Notes 45 kmol/h Decane is cracked into hexane and butane Feed 20 mol/mol% decane 15% of reacted dodecane is cracked into two 80 mol/mol% dodecane hexanes; balance into octane and butane Liquid Hexane Assume decane and dodecane are 100% product Octane cracked (none remain in liquid) Gaseous product Butane Excess H2 Assume no hexane or octane evaporateStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


