Question
A 50.0 g sample of CaCO3 (100.09 g/mol) is reacted with 35.0 g of H3PO4 (97.994 g/mol). 3CaCO3 (S) + 2H3PO4 (aq) >Ca3(PO4)2 (S)
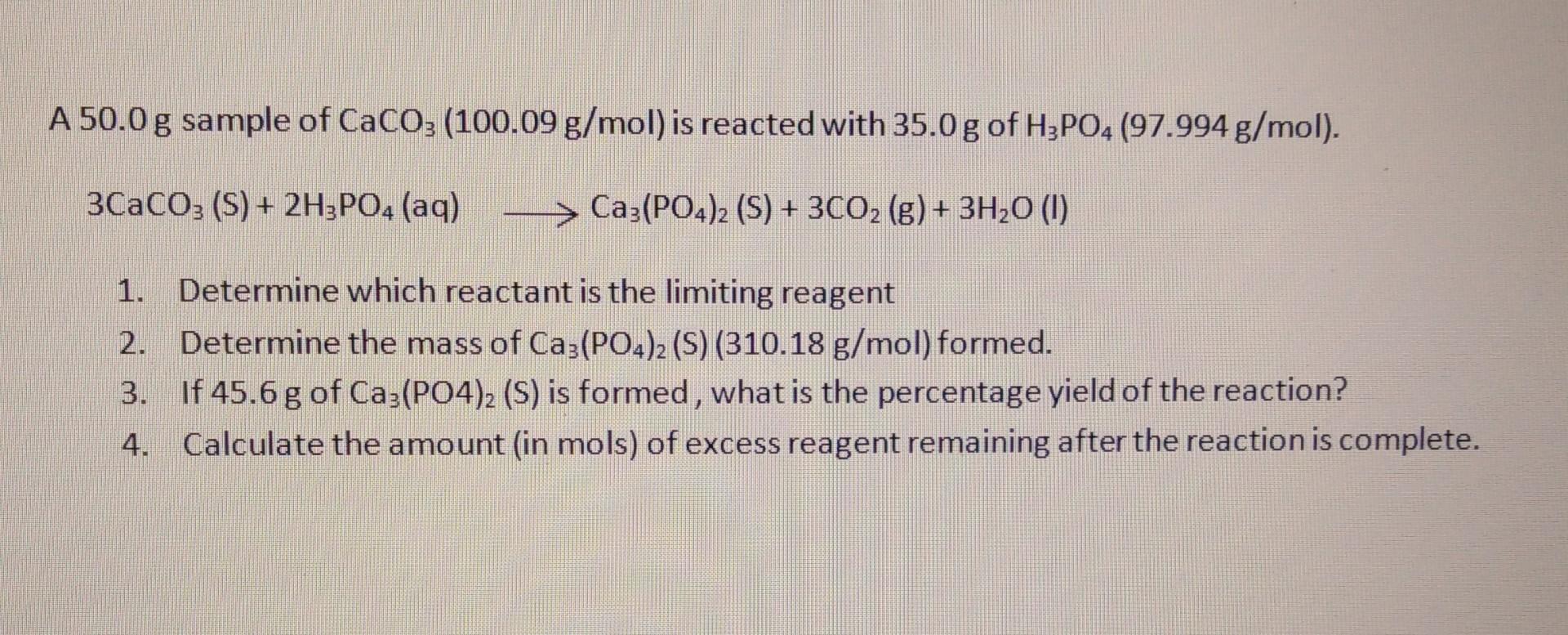
A 50.0 g sample of CaCO3 (100.09 g/mol) is reacted with 35.0 g of H3PO4 (97.994 g/mol). 3CaCO3 (S) + 2H3PO4 (aq) >Ca3(PO4)2 (S) + 3CO (g) + 3HO (1) 1. Determine which reactant is the limiting reagent Determine the mass of Ca3(PO4)2 (S) (310.18 g/mol) formed. If 45.6 g of Ca3(PO4)2 (S) is formed, what is the percentage yield of the reaction? Calculate the amount (in mols) of excess reagent remaining after the reaction is complete. 2. 3.
Step by Step Solution
3.54 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
1 Limiting reagent 3 La Coz s 2H PO aq a s 3 0g 30 1 3 mol 2 mol 3x 10009g 2x 23994 1 95988 30027 g ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Operations Management Creating Value Along the Supply Chain
Authors: Roberta S. Russell, Bernard W. Taylor
7th Edition
9781118139523, 0470525908, 1118139526, 978-0470525906
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



