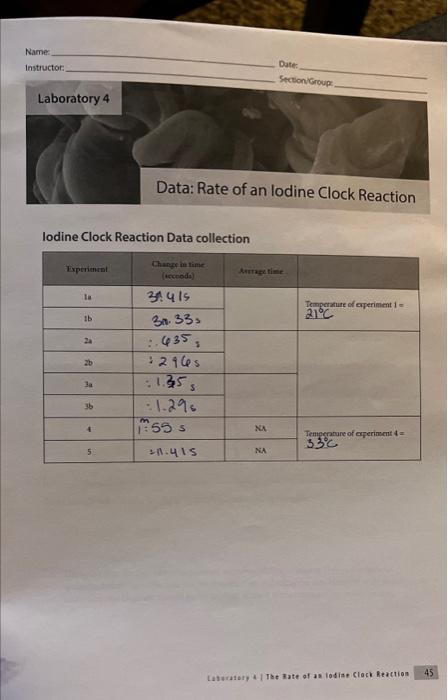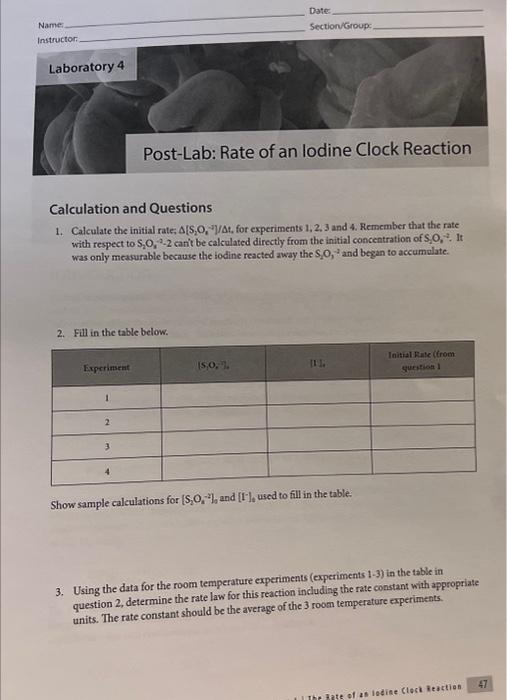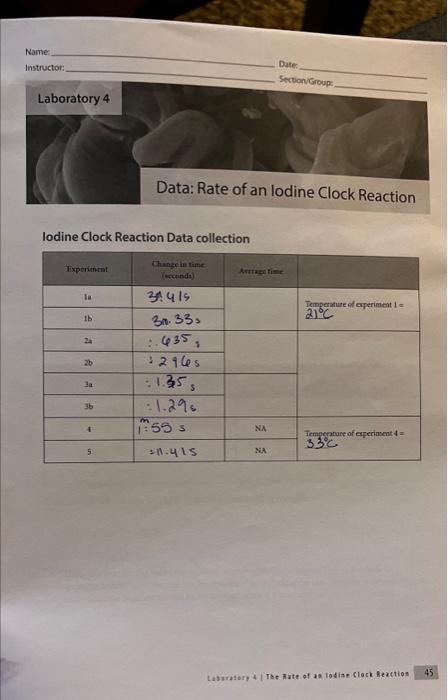
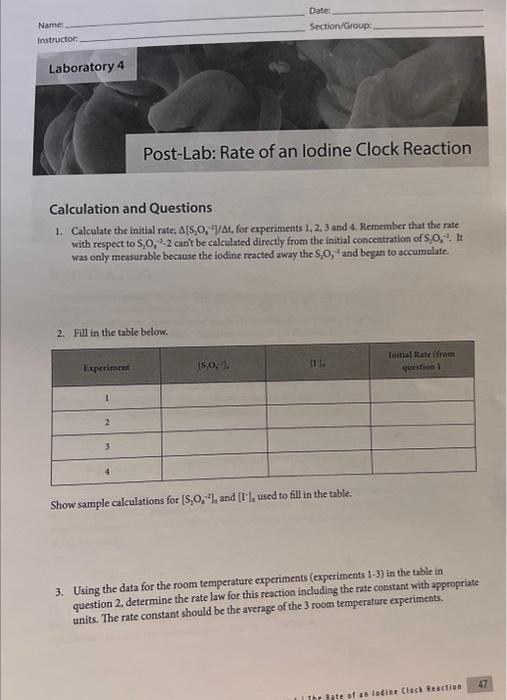

lodine Clock Reaction Data collection Laburabory 4 . The inate of as lodian Clock Reaction Date Name Section/Group: Calculation and Questions 1. Calculate the initial rate; [S2O41]At, for experiments 1,2,3 and 4. Remember that the rate with respect to S2O412 canit be calculated directly from the initial concentration of S2O42. It was only measurable becaure the iodine reacted away the S4O4 and began to accumblate. 2. Fill in the table below. Show sample calculations for [S2O42]3 and [1]6, used to fill in the table. 3. Using the data for the room temperafure experiments (experiments 1-3) in the table in question 2, determine the rate law for this reaction including the rate constant with appropriate units. The rate constant should be the average of the 3 room temperature experiments. 4. Use the rate law to quantitatively explain what would happen in the following situations: a. What would happen to the rate of the reaction if the lodide concentration was doubled? b. What would happen to the rate of the reaction if the persulfate concentration was halved? c. What would happen to the rate of the reaction if the thiosulfate concentration was doubled? d. How would increasing the total volume of the solution affect the actual rate of the iodine clock reaction? 5. Calculate the rate constant at the higher temperature (tube 4). 6. Calculate the activation energy for the reaction. 7. Describe the effect that the catalyst had on the rate of the reaction. lodine Clock Reaction Data collection Lastaiory i I Toe 3ate of as lodiae Clock feaction 45 Date Name: Section-Group: Calculation and Questions 1. Calculate the initial rate: [S2O1,]/t, for experiments 1, 2,3 and 4. Remember that the rate with respect to S2O322 canit be calculated directly from the initial concentration of S2O42. It was only mearurable because the iodine reacted away the S2O2 and began to accumolate. 2. Fill in the table below. Show sample calculations for [S2O42]3 and [T6]6 used to fill in the table. 3. Using the data for the room temperature experiments (experiments 1-3) in the table in question 2, determine the rate law for this reaction including the rate constant with appropriate units. The rate constant should be the average of the 3 room temperature experiments. 4. Use the rate law to quantitatively explain what would happen in the following situations: a. What would happen to the rate of the reaction if the lodide concentration was doubled? b. What would happen to the rate of the reaction if the persulfate concentration was halved? c. What would happen to the rate of the reaction if the thiosulfate concentration was doubled? d. How would increasing the total volume of the solution affect the actual rate of the iodine clock reaction? 5. Calculate the rate constant at the higher temperature (tube 4). 6. Calculate the activation energy for the reaction. 7. Describe the effect that the catalyst had on the rate of the reaction. lodine Clock Reaction Data collection Laburabory 4 . The inate of as lodian Clock Reaction Date Name Section/Group: Calculation and Questions 1. Calculate the initial rate; [S2O41]At, for experiments 1,2,3 and 4. Remember that the rate with respect to S2O412 canit be calculated directly from the initial concentration of S2O42. It was only measurable becaure the iodine reacted away the S4O4 and began to accumblate. 2. Fill in the table below. Show sample calculations for [S2O42]3 and [1]6, used to fill in the table. 3. Using the data for the room temperafure experiments (experiments 1-3) in the table in question 2, determine the rate law for this reaction including the rate constant with appropriate units. The rate constant should be the average of the 3 room temperature experiments. 4. Use the rate law to quantitatively explain what would happen in the following situations: a. What would happen to the rate of the reaction if the lodide concentration was doubled? b. What would happen to the rate of the reaction if the persulfate concentration was halved? c. What would happen to the rate of the reaction if the thiosulfate concentration was doubled? d. How would increasing the total volume of the solution affect the actual rate of the iodine clock reaction? 5. Calculate the rate constant at the higher temperature (tube 4). 6. Calculate the activation energy for the reaction. 7. Describe the effect that the catalyst had on the rate of the reaction. lodine Clock Reaction Data collection Lastaiory i I Toe 3ate of as lodiae Clock feaction 45 Date Name: Section-Group: Calculation and Questions 1. Calculate the initial rate: [S2O1,]/t, for experiments 1, 2,3 and 4. Remember that the rate with respect to S2O322 canit be calculated directly from the initial concentration of S2O42. It was only mearurable because the iodine reacted away the S2O2 and began to accumolate. 2. Fill in the table below. Show sample calculations for [S2O42]3 and [T6]6 used to fill in the table. 3. Using the data for the room temperature experiments (experiments 1-3) in the table in question 2, determine the rate law for this reaction including the rate constant with appropriate units. The rate constant should be the average of the 3 room temperature experiments. 4. Use the rate law to quantitatively explain what would happen in the following situations: a. What would happen to the rate of the reaction if the lodide concentration was doubled? b. What would happen to the rate of the reaction if the persulfate concentration was halved? c. What would happen to the rate of the reaction if the thiosulfate concentration was doubled? d. How would increasing the total volume of the solution affect the actual rate of the iodine clock reaction? 5. Calculate the rate constant at the higher temperature (tube 4). 6. Calculate the activation energy for the reaction. 7. Describe the effect that the catalyst had on the rate of the reaction