Answered step by step
Verified Expert Solution
Question
1 Approved Answer
lodine-123 is a radioactive isotope used in medicine to test the function of the thyroid gland. Its half-life is 13.1 hours. A 575-microcurie (uCi)
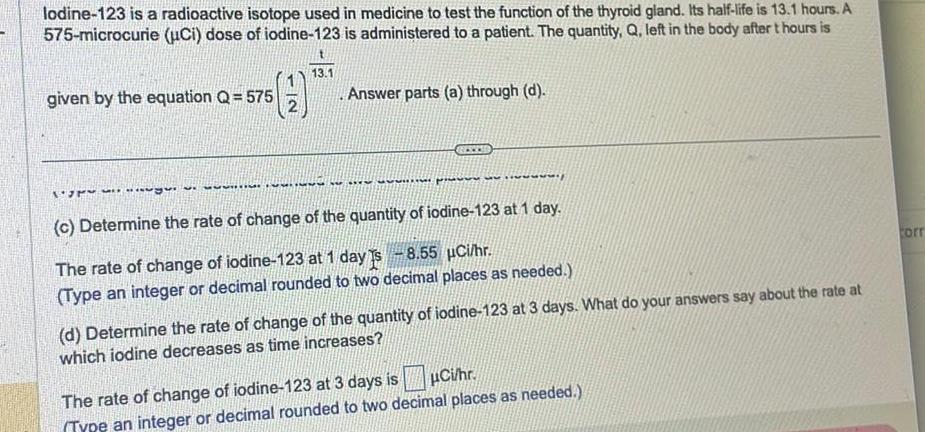
lodine-123 is a radioactive isotope used in medicine to test the function of the thyroid gland. Its half-life is 13.1 hours. A 575-microcurie (uCi) dose of iodine-123 is administered to a patient. The quantity, Q, left in the body after t hours is given by the equation Q = 575 13.1 .Answer parts (a) through (d). (c) Determine the rate of change of the quantity of iodine-123 at 1 day. The rate of change of iodine-123 at 1 day Is -8.55 Ci/hr. (Type an integer or decimal rounded to two decimal places as needed.) (d) Determine the rate of change of the quantity of iodine-123 at 3 days. What do your answers say about the rate at which iodine decreases as time increases? The rate of change of iodine-123 at 3 days is Ci/hr. (Type an integer or decimal rounded to two decimal places as needed.) corr
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


