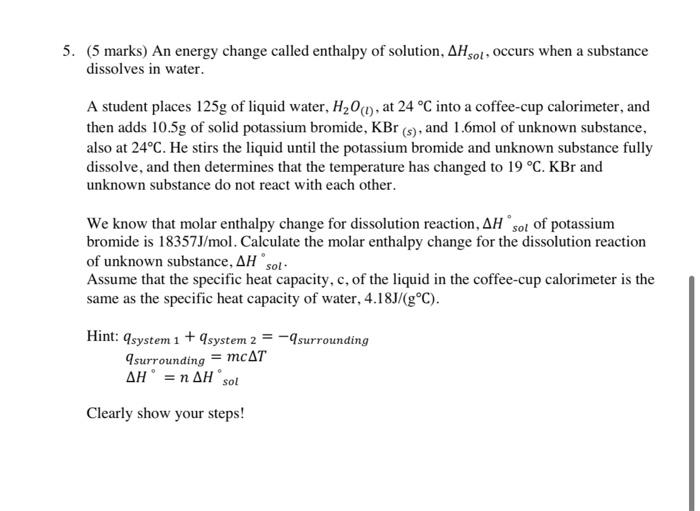
Long Answers: 1. (4 marks) Use either standard enthalpies of formation or bond energies to calculate the enthalpy change of the following chemical reaction. CH2OHo) +020) + CO2(9) + H209 2. (4 marks) Consider the following thermochemical equations: 2 N269) + 2 H269) NH3(9) AH = -46K) 3M2() +C126) - HCI AH = -92K) N269) + 4H269) + C12(9) 2NH,CH) AH = -628K) Use Hess's Law to calculate AH for the reaction represented by the following equation representing the formation of ammonium chloride. NH3(9) + HCL) - NH.CH 3. (5 marks) For the theoretical reaction represented by the balanced chemical equation 2A+B+C - 2D + E Run Initial (A) Initial [B] initial [C] Rate (mol/L) (mol/L) (mol/L) 1 0.025 0.035 0.022 0.0060 2 0.025 0.035 0.044 0.012 3 0.025 0.140 0.022 0.0060 4 0.050 0.035 0.044 0.048 a) (3 marks) Determine the order of the reaction with respect to each reactant. Explain HOW do you get order for each reactant. b) (2 marks) Write the specific rate law equation for the reaction. Making sure to include magnitude and unit fork. Show steps. 4. (4 marks) A student wishes to increase the rate of the following reaction: H2SO4(aq) + NaOH(aq) + H20) + Na2SO4(20) Identify two different ways and use collision theory to explain your choice. 5. (5 marks) An energy change called enthalpy of solution, AH sol, occurs when a substance dissolves in water. A student places 125g of liquid water, H20 (1), at 24 C into a coffee-cup calorimeter, and then adds 10.5g of solid potassium bromide, KBr (s), and 1.6mol of unknown substance, also at 24C. He stirs the liquid until the potassium bromide and unknown substance fully dissolve, and then determines that the temperature has changed to 19 C. KBr and unknown substance do not react with each other. We know that molar enthalpy change for dissolution reaction, AH sol of potassium bromide is 18357J/mol. Calculate the molar enthalpy change for the dissolution reaction of unknown substance, AH sot Assume that the specific heat capacity, c, of the liquid in the coffee-cup calorimeter is the same as the specific heat capacity of water, 4.18J/(gC). Hint: qsystem 1 + 9system 2 = -9 surrounding 9surrounding = mcAT AH = n AH sol Clearly show your steps