Answered step by step
Verified Expert Solution
Question
1 Approved Answer
looking for B,C, and D Given that the equilarium constant K=21.96 at 672K, we are given initial concentratiors, and we are askod lor For the
looking for B,C, and D 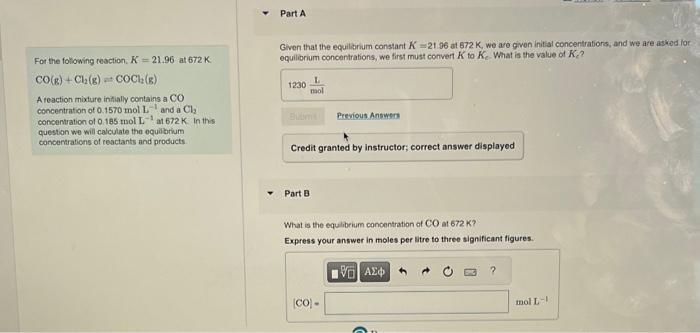
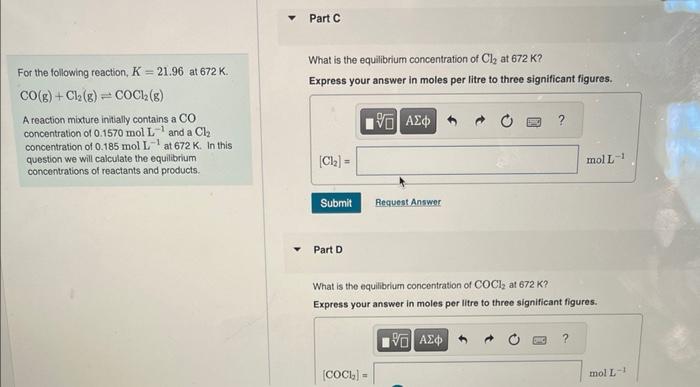
Given that the equilarium constant K=21.96 at 672K, we are given initial concentratiors, and we are askod lor For the folowing reaction. K=21.96 at 672K. equiliorium concentrations, we first must convert K to Ke. What is the value of Ke ? CO(g)+Cl2(g)COCl2(g) A feaction mixture inifially contains a CO concentration of 0.1570molL1 and a Cl2 concentration of 0.185 wol L1 at 672K. In the question we will calculate the equilbrium concentraticns of reactants and products. Credit granted by instructor; correct answer displayed Part B What is the equlbeium concentration of CO at 672K ? Express your answer in moles per litre to three significant figures. For the following reaction, K=21.96 at 672K. What is the equilibrium concentration of Cl2 at 672K ? CO(g)+Cl2(g)COCl2(g) Express your answer in moles per litre to three significant figures. A reaction mixture initially contains a CO concentration of 0.1570molL1 and aCl2 concentration of 0.185molL1 at 672K. In this question we will calculate the equilibrium concentrations of reactants and products. Part D What is the equilibrium concentration of COCl2 at 672K ? Express your answer in moles per litre to three significant figures 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


