Answered step by step
Verified Expert Solution
Question
1 Approved Answer
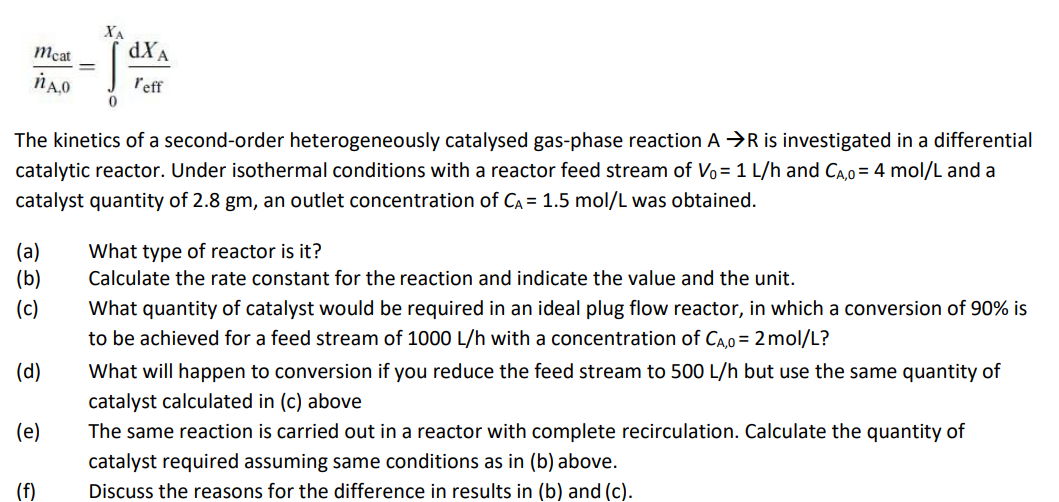
m c a t n A , 0 = 0 x A d x A r e f f The kinetics of a second -
The kinetics of a secondorder heterogeneously catalysed gasphase reaction is investigated in a differential
catalytic reactor. Under isothermal conditions with a reactor feed stream of and and a
catalyst quantity of an outlet concentration of was obtained.
a What type of reactor is it
b Calculate the rate constant for the reaction and indicate the value and the unit.
c What quantity of catalyst would be required in an ideal plug flow reactor, in which a conversion of is
to be achieved for a feed stream of with a concentration of
d What will happen to conversion if you reduce the feed stream to but use the same quantity of
catalyst calculated in c above
e The same reaction is carried out in a reactor with complete recirculation. Calculate the quantity of
catalyst required assuming same conditions as in b above.
f Discuss the reasons for the difference in results in b and c
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


