Answered step by step
Verified Expert Solution
Question
1 Approved Answer
m An industrial reactor needs 7530 of methanol-nitrogen mixture at 100 C and the dew h point 48 C to carry out a catalytic
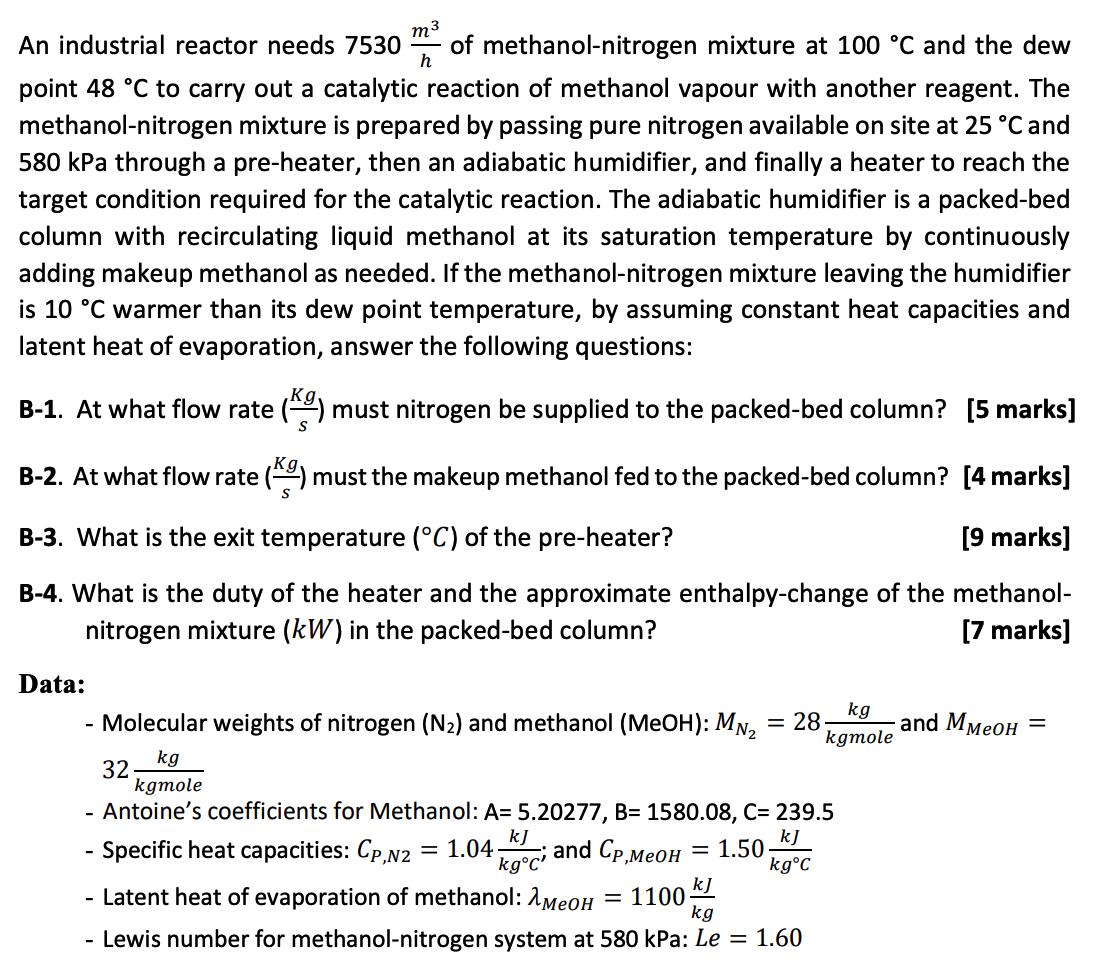
m An industrial reactor needs 7530 of methanol-nitrogen mixture at 100 C and the dew h point 48 C to carry out a catalytic reaction of methanol vapour with another reagent. The methanol-nitrogen mixture is prepared by passing pure nitrogen available on site at 25 C and 580 kPa through a pre-heater, then an adiabatic humidifier, and finally a heater to reach the target condition required for the catalytic reaction. The adiabatic humidifier is a packed-bed column with recirculating liquid methanol at its saturation temperature by continuously adding makeup methanol as needed. If the methanol-nitrogen mixture leaving the humidifier is 10 C warmer than its dew point temperature, by assuming constant heat capacities and latent heat of evaporation, answer the following questions: B-1. At what flow rate ( must nitrogen be supplied to the packed-bed column? [5 marks] S B-2. At what flow rate () must the makeup methanol fed to the packed-bed column? [4 marks] B-3. What is the exit temperature (C) of the pre-heater? [9 marks] B-4. What is the duty of the heater and the approximate enthalpy-change of the methanol- nitrogen mixture (kW) in the packed-bed column? [7 marks] Data: - Molecular weights of nitrogen (N) and methanol (MeOH): MN - 28 kg 32 kgmole - Antoine's coefficients for Methanol: A= 5.20277, B= 1580.08, C= 239.5 - Specific heat capacities: Cp,N2 1.04. and Cp, kJ = 1.50. kgC kJ - Latent heat of evaporation of methanol: MeOH = 1100- Lewis number for methanol-nitrogen system at 580 kPa: Le = 1.60 kg = kg kgmole kJ kgC' and M =
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
3 1 B ka must nitragen be supplied to the packedbed column if ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


