Question
Many common acids are 'oxyacids', i.e. the 'acidic' hydrogen is covalently bound to an oxygen atom. Key Questions: 7.1) Besides HClO4, there are five
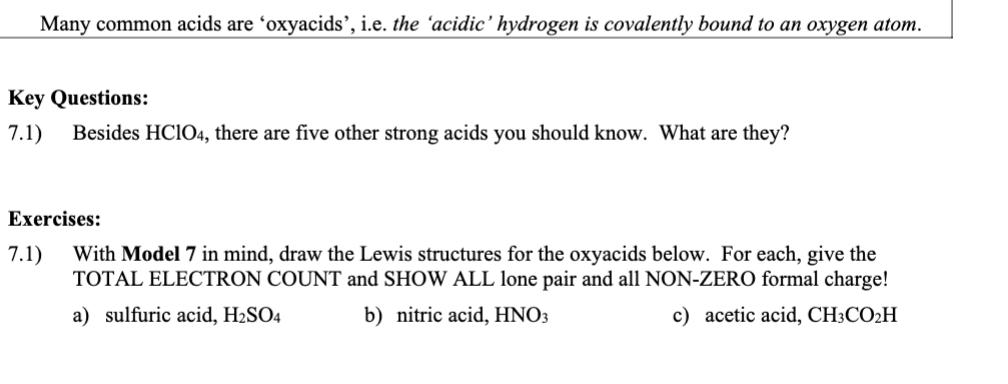
Many common acids are 'oxyacids', i.e. the 'acidic' hydrogen is covalently bound to an oxygen atom. Key Questions: 7.1) Besides HClO4, there are five other strong acids you should know. What are they? Exercises: 7.1) With Model 7 in mind, draw the Lewis structures for the oxyacids below. For each, give the TOTAL ELECTRON COUNT and SHOW ALL lone pair and all NON-ZERO formal charge! a) sulfuric acid, H2SO4 c) acetic acid, CH3COH b) nitric acid, HNO3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essential Cell Biology
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter
5th Edition
0393680363, 978-0393680362
Students also viewed these Algorithms questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



