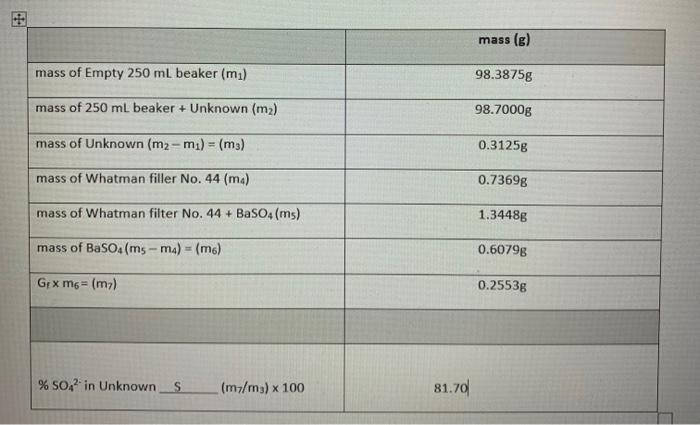
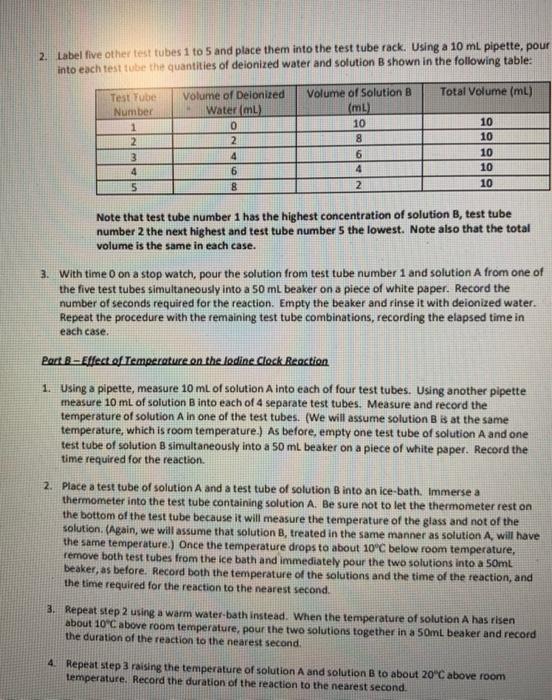
mass (g) mass of Empty 250 ml beaker (m) 98.3875g mass of 250 ml beaker + Unknown (m2) 98.7000g mass of Unknown (m2-m) = (mg) 0.3125g mass of Whatman filler No. 44 (ma) 0.73698 mass of Whatman filter No.44 + BaSO4(ms) 1.34486 mass of BaSO4 (ms - ma) = (m6) 0.60798 GpXm6= (m2) 0.25538 % SO42 in Unknown S (m/m) x 100 81.70 Experiment 8: A Study of Reaction Rates Introduction: The word rate implies change and time-change in some measurable quantity and the interval of time over which the change occurs. Speed in kilometres per hour, salary in dollars per month, and quantity in litres per minute all express the idea of rate. The rate of a chemical reaction tells us how fast a reactant is being consumed or a product is being produced. Specifically, rate of reaction is the positive quantity that denotes how the concentration of a species in the reaction changes with time. For example, in the reaction N,Co) + 3H,() - 2N7,00) the rate of the reaction can be expressed in the forward direction, in terms of a product: change in concentration of NH, Rate = time interval Alternatively, the rate could be expressed in the reverse direction, in terms of a reactant: change in concentration of N2 Rate = time interval The negative sign is necessary to make the rate a positive quantity since the concentration of the reactant, N, is decreasing with time. The rate of a chemical reaction can be changed by (a) varying the concentration of reactants, (b) changing the temperature, (c) introducing a catalyst, or (d) changing the surface area of contact between reactants. Catalysts are substances that drastically alter reaction rates without being used up in the reaction. In this experiment, students will study the effect of concentration by varying the concentration of one reactant while holding others constant, examine the rates of the same reaction at different temperatures, and conduct two reactions that will be identical in all respects except that a catalyst will be present in once and not in the other. Student will not be examining the concept of altering surface area between reactants, though, in this case, the rate of reaction increases by maximizing the surface area of contact between reactants. In analyzing the results of this experiment, time of reaction will be used as an indication of rate. Time and rate are inversely related the higher the rate, the shorter the time. This is evident if you think of driving from one location to another it takes less time if you drive at a higher rate fspeed). The principal reaction whose rate will be studied in this experiment is the iodine "clock reaction. It is the reaction between two solutions, solution A (0.02 M potassium iodate, KID,) and solution B (0.002 M sodium bisulphite, NaHSO). When the two solutions are combined, a series of reactions begin ending with the release of elemental lodine. The appearance of iodine in the presence of starch may be detected easily by the appearance of a deep blue colour. This signals the completion of the reaction Asafety Precautions Sulphuric acid can cause severe burns when in contact with skin. If you should spill any of the 6M acid on yourself, immediately wash it off with large amounts of cold water. Beware that sulphuric acid generates a lot of heat when it comes in contact with water. Wear safety goggles, a lab coat, and latex gloves when performing this experiment. Solutions obtained in this experiment may be disposed of down the drain. Reagents: 6 MH.SO. (Sulphuric acid) 0.02 M KIO. (Potassium lodate) 0.002 M NaHSO. (Sodium bisulphite) 1 M CuSO. (Copper il sulfate) De-lonized Water (DI) Equipment: ( 16 ) Medium Test tubes (5) 50 mL Beaker (1) 600 mL Beaker 11) Thermometer (3) Serological Pipettes (1) Vortex (1) Hot plate Procedure: Part A - Effect of Concentration on the lodine Clock Reaction To study the effect of concentration on the reaction rate, you will keep total volume, concentration of solution A, and temperature constant, and vary only the concentration of solution B. 1. Pour 10 mL of solution A into five labelled test tubes with a 10 ml pipette. Place the test tubes into a test tube rack. 2. Label five other test tubes 1 to 5 and place them into the test tube rack. Using a 10 ml pipette, pour into each test tube the quantities of deionized water and solution B shown in the following table: Total Volume (mL) Test Tube Number 1 2 3 4 Volume of Delonized Water (mL) 0 2 4 6 8 Volume of Solution B (mL) 10 8 6 4 2 10 10 10 10 10 5 Note that test tube number 1 has the highest concentration of solution B, test tube number 2 the next highest and test tube number the lowest. Note also that the total volume is the same in each case. 3. With time on a stop watch, pour the solution from test tube number 1 and solution A from one of the five test tubes simultaneously into a 50 ml beaker on a piece of white paper. Record the number of seconds required for the reaction. Emply the beaker and rinse it with deionized water. Repeat the procedure with the remaining test tube combinations, recording the elapsed time in each case. Part 8 - Effect of Temperature on the ledine Clock Reaction 1. Using a pipette, measure 10 mL of solution A into each of four test tubes. Using another pipette measure 10 mL of solution B into each of 4 separate test tubes. Measure and record the temperature of solution A in one of the test tubes. (We will assume solution B is at the same temperature, which is room temperature.) As before, empty one test tube of solution A and one test tube of solution 8 simultaneously into a 50 ml beaker on a piece of white paper. Record the time required for the reaction. 2. Place a test tube of solution A and a test tube of solution B into an ice-bath. Immerse a thermometer into the test tube containing solution A. Be sure not to let the thermometer rest on the bottom of the test tube because it will measure the temperature of the glass and not of the solution. (Again, we will assume that solution B, treated in the same manner as solution A, will have the same temperature. Once the temperature drops to about 10C below room temperature, remove both test tubes from the ice bath and immediately pour the two solutions into a 50ml beaker, as before. Record both the temperature of the solutions and the time of the reaction, and the time required for the reaction to the nearest second 3. Repeat step 2 using a warm water-bath instead. When the temperature of solution A has risen 2 about 10"C above room temperature, pour the two solutions together in a 50ml beaker and record the duration of the reaction to the nearest secon 4. Repeat step 3 raising the temperature of solution A and solution B to about 20C above room temperature. Record the duration of the reaction to the nearest second. Part C-Effect of a Catalyst on the lodine Clock Reaction 1. Measure 10mL of solution A into a test tube and add a drop of 10 M copper(ll) sulphate, Cuso,, a catalyst. In another test tube, combine 2mL of solution B and 8mL of deionized water (the same procedure as in Part A test tube number 4). Pour the solutions simultaneously into a 50ml beaker and record the time required for reaction. 2. RINSE ALL TEST TUBES THOROUGHLY WITH DENIONIZED WATER AND AIR DRY THEM INVERTED IN TEST TUBE RACKS ON THE CART PROVIDED. RINSE ALL OTHER GLASSWARE USED AND RETURN THEM TO THEIR APPROPRIATE LOCATION