Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Mass percent #1 The contribution (expressed as a %) of a given element to the mass of a compound is called a mass percent.
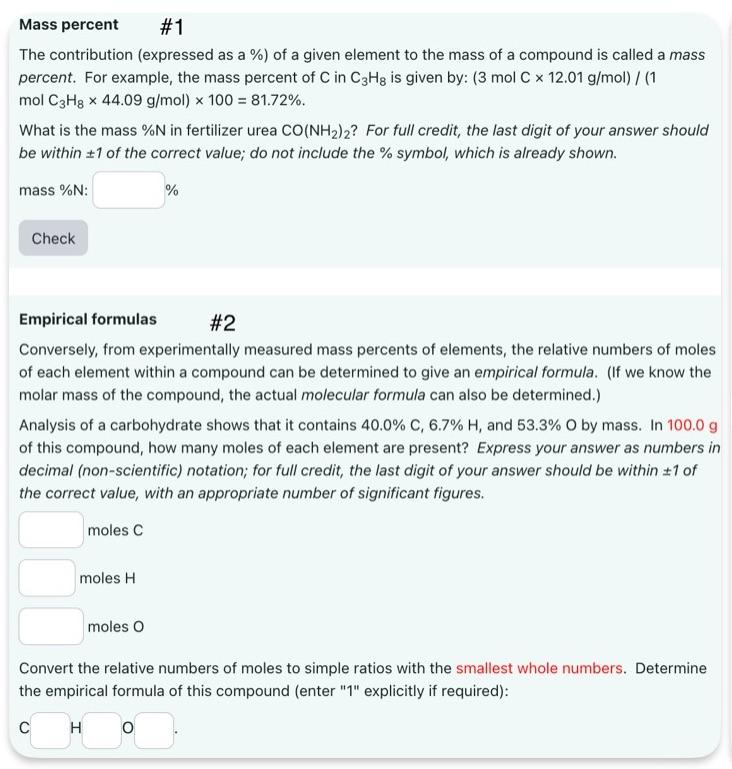
Mass percent #1 The contribution (expressed as a %) of a given element to the mass of a compound is called a mass percent. For example, the mass percent of C in C3Hg is given by: (3 mol C x 12.01 g/mol) / (1 mol C3H8 x 44.09 g/mol) x 100 = 81.72%. What is the mass %N in fertilizer urea CO(NH2)2? For full credit, the last digit of your answer should be within 1 of the correct value; do not include the % symbol, which is already shown. mass %N: Check % Empirical formulas #2 Conversely, from experimentally measured mass percents of elements, the relative numbers of moles of each element within a compound can be determined to give an empirical formula. (If we know the molar mass of the compound, the actual molecular formula can also be determined.) Analysis of a carbohydrate shows that it contains 40.0% C, 6.7% H, and 53.3% O by mass. In 100.0 g of this compound, how many moles of each element are present? Express your answer as numbers in decimal (non-scientific) notation; for full credit, the last digit of your answer should be within 1 of the correct value, with an appropriate number of significant figures. moles C moles H moles O Convert the relative numbers of moles to simple ratios with the smallest whole numbers. Determine the empirical formula of this compound (enter "1" explicitly if required): C H
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


