Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Mass transfer It is desired to remove 90% of SO2 in a flue gas stream at 298 K and 1 atm by countercurrent absorption with
Mass transfer
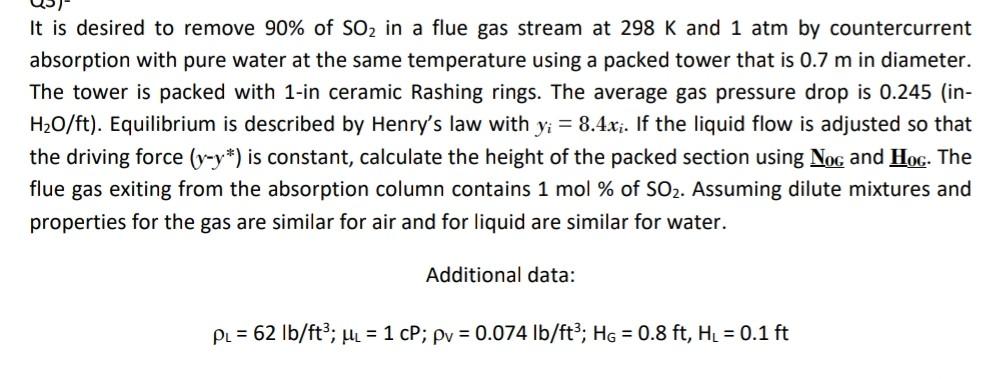
It is desired to remove 90% of SO2 in a flue gas stream at 298 K and 1 atm by countercurrent absorption with pure water at the same temperature using a packed tower that is 0.7 m in diameter. The tower is packed with 1-in ceramic Rashing rings. The average gas pressure drop is 0.245 (in- H2O/ft). Equilibrium is described by Henry's law with yi = 8.4x;. If the liquid flow is adjusted so that the driving force (y-y*) is constant, calculate the height of the packed section using Nog and Hog. The flue gas exiting from the absorption column contains 1 mol % of SO2. Assuming dilute mixtures and properties for the gas are similar for air and for liquid are similar for water. Additional data: PL = 62 lb/ft?; ML = 1 CP; Py = 0.074 lb/ft?; Hg = 0.8 ft, HL = 0.1 ftStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


