Question
m-Dinitrobenzene melts at 89.8C at I atm and 114.8C at 968 atm. If its heat of fusion is 174 KJ/mole, what is the increase
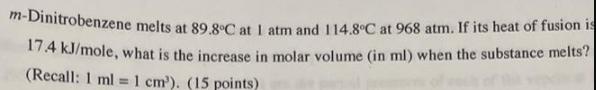
m-Dinitrobenzene melts at 89.8C at I atm and 114.8C at 968 atm. If its heat of fusion is 174 KJ/mole, what is the increase in molar volume (in ml) when the substance melts? (Recall: 1 ml =1 cm). (15 points) %3D
Step by Step Solution
3.47 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
2 The following relationship shall be used to calculate appr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Foundations of Macroeconomics
Authors: Robin Bade, Michael Parkin
8th edition
134492005, 978-0134492001
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



