Answered step by step
Verified Expert Solution
Question
1 Approved Answer
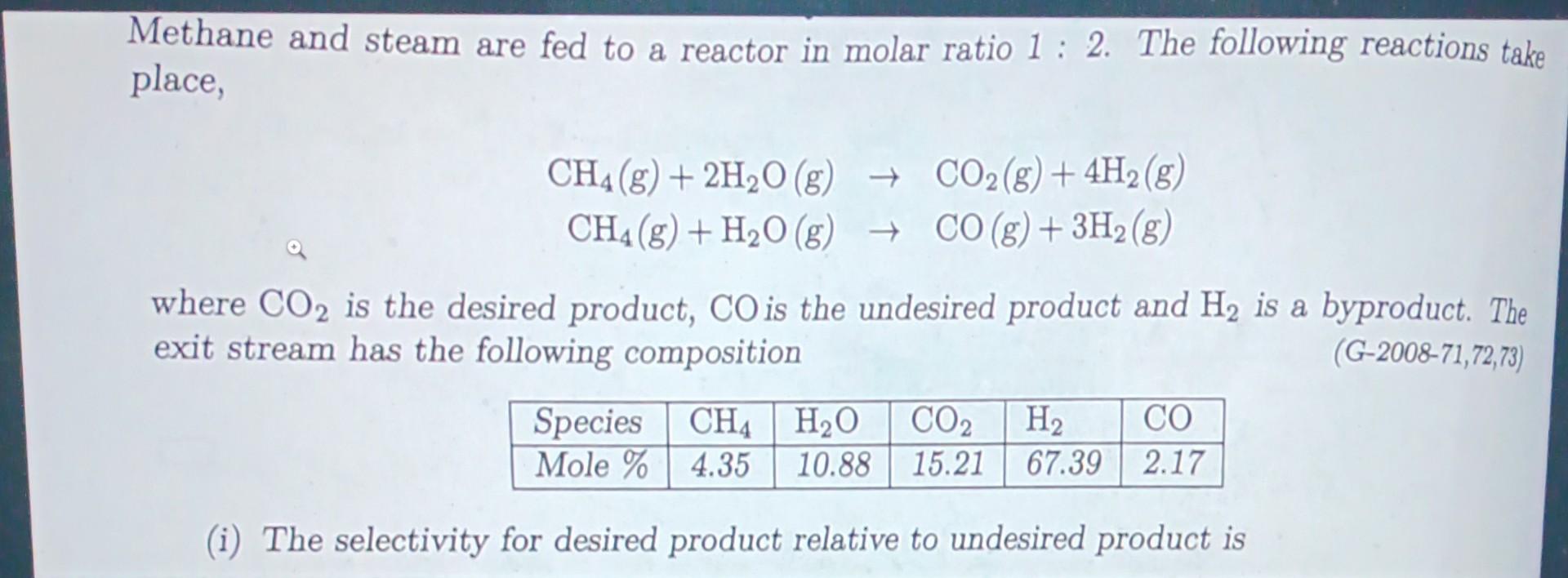
Methane and steam are fed to a reactor in molar ratio 1 : 3 . The following reactions take place, CH 4 ( 8 )
Methane and steam are fed to a reactor in molar ratio : The following reactions take place, CHHOg COgH CH HOg COgH where CO is the desired product, CO is the undesired product and H is a byproduct. The exit stream has the following composition G Species CH HO CO H CO Mole i The selectivity for desired product relative to undesired product is ii The fractional yield of CO is where fractional yield is defined as the ratio of moles of the desired product formed to the moles that would have been formed if there were no side reactions and the limiting reactant had reacted completelyiii The fractional conversion of methane is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


