Question
Methane burns in air by the following chemical reaction: This reaction can be idealized as a constant pressure or a constant volume process. Calculate the
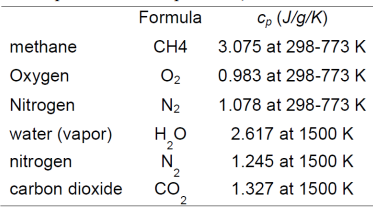
Methane burns in air by the following chemical reaction: This reaction can be idealized as a constant pressure or a constant volume process. Calculate the maximum temperatures of combustion for these two cases. Assume that the specific heats are constant for the temperature range of interest (use the value at 1500 K). Also assume that methane reacts with a stoichiometric amount of air and that the methane and air are initially at 500C and 10 atm. The heat of combustion at 25C for one kg of methane burned (lower heat value) is -50,010 kJ. (Note that no enthalpy of formation is given for the reactants and products in this problem.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


