Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Methane diffuses at steady state through a tube containing helium. At point 1 the partial pressure of methane is pAl=65kPa and at point 2,0.03m apart
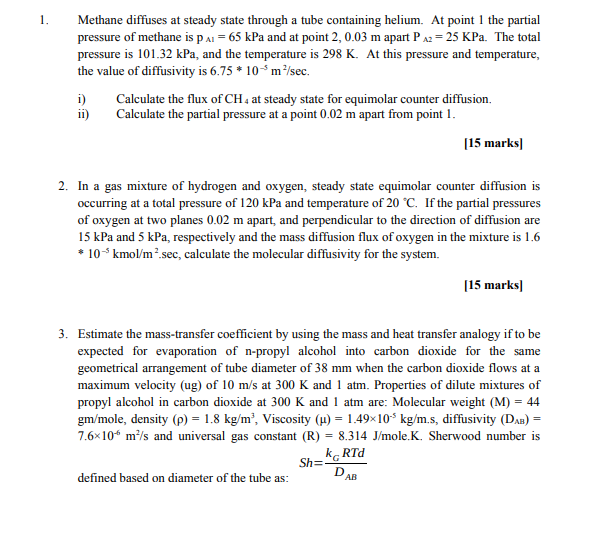 Methane diffuses at steady state through a tube containing helium. At point 1 the partial pressure of methane is pAl=65kPa and at point 2,0.03m apart PA2=25KPa. The total pressure is 101.32kPa, and the temperature is 298K. At this pressure and temperature, the value of diffusivity is 6.75105m2/sec. i) Calculate the flux of CH4 at steady state for equimolar counter diffusion. ii) Calculate the partial pressure at a point 0.02m apart from point 1 . [15 marks] 2. In a gas mixture of hydrogen and oxygen, steady state equimolar counter diffusion is occurring at a total pressure of 120kPa and temperature of 20C. If the partial pressures of oxygen at two planes 0.02m apart, and perpendicular to the direction of diffusion are 15kPa and 5kPa, respectively and the mass diffusion flux of oxygen in the mixture is 1.6 105kmol/m2.sec, calculate the molecular diffusivity for the system. [15 marks] 3. Estimate the mass-transfer coefficient by using the mass and heat transfer analogy if to be expected for evaporation of n-propyl alcohol into carbon dioxide for the same geometrical arrangement of tube diameter of 38mm when the carbon dioxide flows at a maximum velocity (ug) of 10m/s at 300K and 1atm. Properties of dilute mixtures of propyl alcohol in carbon dioxide at 300K and 1atm are: Molecular weight (M)=44 gm/mole, density ()=1.8kg/m3, Viscosity ()=1.49105kg/m.s, diffusivity (DAB)= 7.6106m2/s and universal gas constant (R)=8.314J/mole.K. Sherwood number is defined based on diameter of the tube as: Sh=DABkGRTd
Methane diffuses at steady state through a tube containing helium. At point 1 the partial pressure of methane is pAl=65kPa and at point 2,0.03m apart PA2=25KPa. The total pressure is 101.32kPa, and the temperature is 298K. At this pressure and temperature, the value of diffusivity is 6.75105m2/sec. i) Calculate the flux of CH4 at steady state for equimolar counter diffusion. ii) Calculate the partial pressure at a point 0.02m apart from point 1 . [15 marks] 2. In a gas mixture of hydrogen and oxygen, steady state equimolar counter diffusion is occurring at a total pressure of 120kPa and temperature of 20C. If the partial pressures of oxygen at two planes 0.02m apart, and perpendicular to the direction of diffusion are 15kPa and 5kPa, respectively and the mass diffusion flux of oxygen in the mixture is 1.6 105kmol/m2.sec, calculate the molecular diffusivity for the system. [15 marks] 3. Estimate the mass-transfer coefficient by using the mass and heat transfer analogy if to be expected for evaporation of n-propyl alcohol into carbon dioxide for the same geometrical arrangement of tube diameter of 38mm when the carbon dioxide flows at a maximum velocity (ug) of 10m/s at 300K and 1atm. Properties of dilute mixtures of propyl alcohol in carbon dioxide at 300K and 1atm are: Molecular weight (M)=44 gm/mole, density ()=1.8kg/m3, Viscosity ()=1.49105kg/m.s, diffusivity (DAB)= 7.6106m2/s and universal gas constant (R)=8.314J/mole.K. Sherwood number is defined based on diameter of the tube as: Sh=DABkGRTd Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


