Answered step by step
Verified Expert Solution
Question
1 Approved Answer
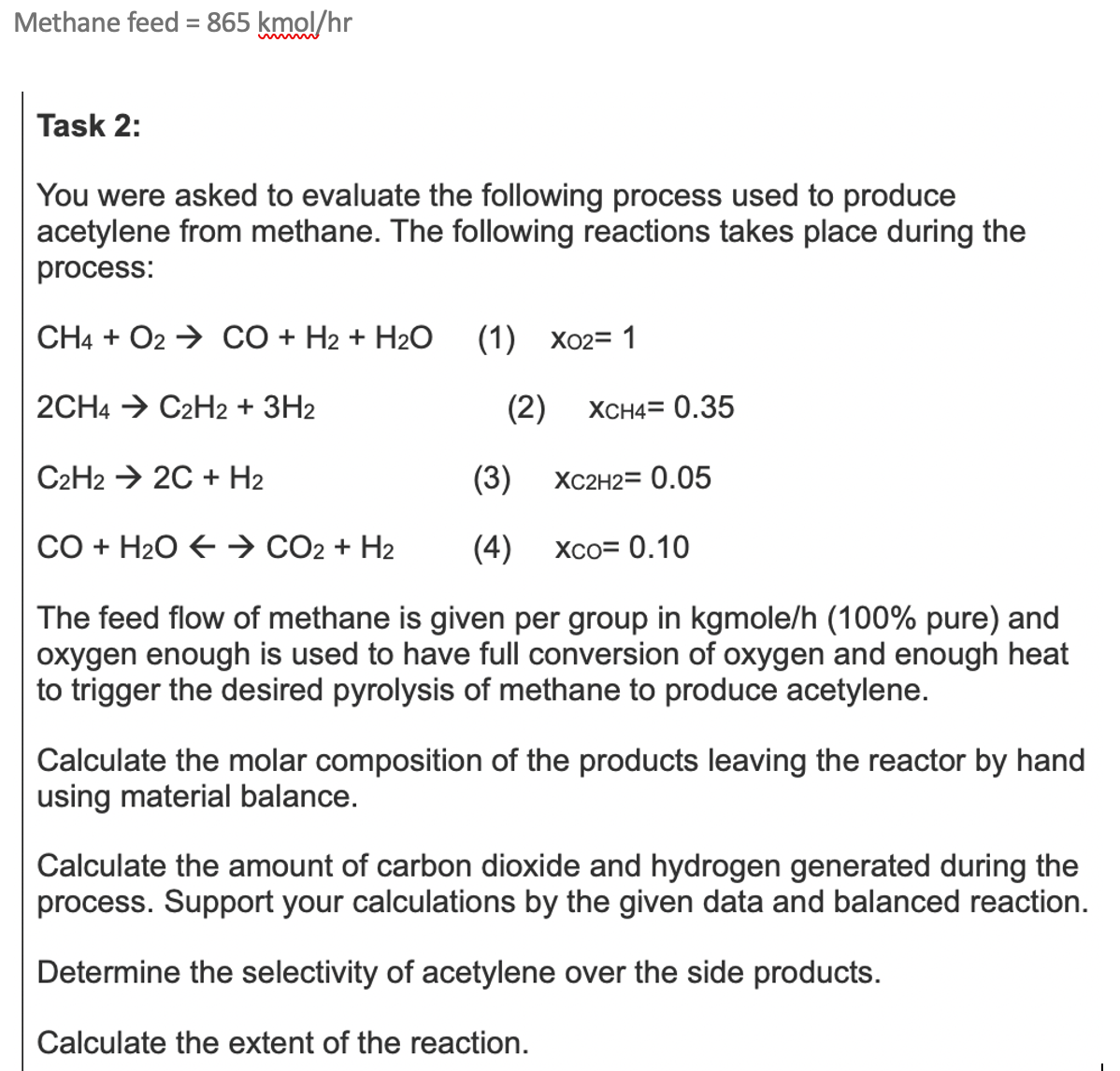
Methane feed = 8 6 5 kmo l h r Task 2 : You were asked to evaluate the following process used to produce acetylene
Methane feed kmo
Task :
You were asked to evaluate the following process used to produce
acetylene from methane. The following reactions takes place during the
process:
Olarr
The feed flow of methane is given per group in kgmoleh pure and
oxygen enough is used to have full conversion of oxygen and enough heat
to trigger the desired pyrolysis of methane to produce acetylene.
Calculate the molar composition of the products leaving the reactor by hand
using material balance.
Calculate the amount of carbon dioxide and hydrogen generated during the
process. Support your calculations by the given data and balanced reaction.
Determine the selectivity of acetylene over the side products.
Calculate the extent of the reaction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


