Answered step by step
Verified Expert Solution
Question
1 Approved Answer
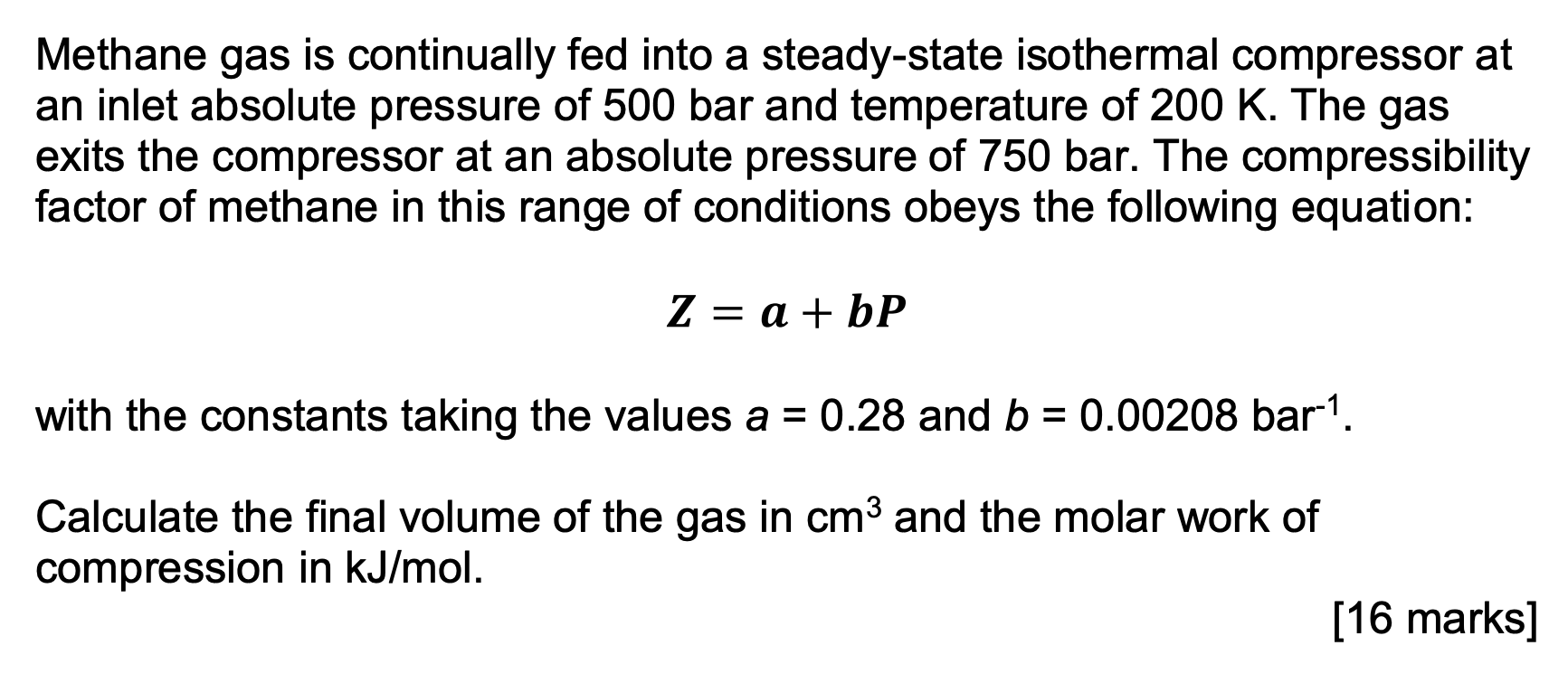
Methane gas is continually fed into a steady - state isothermal compressor at an inlet absolute pressure of 5 0 0 bar and temperature of
Methane gas is continually fed into a steadystate isothermal compressor at
an inlet absolute pressure of bar and temperature of The gas
exits the compressor at an absolute pressure of bar. The compressibility
factor of methane in this range of conditions obeys the following equation:
with the constants taking the values and bar
Calculate the final volume of the gas in and the molar work of
compression in the answer should be kjmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


