Answered step by step
Verified Expert Solution
Question
1 Approved Answer
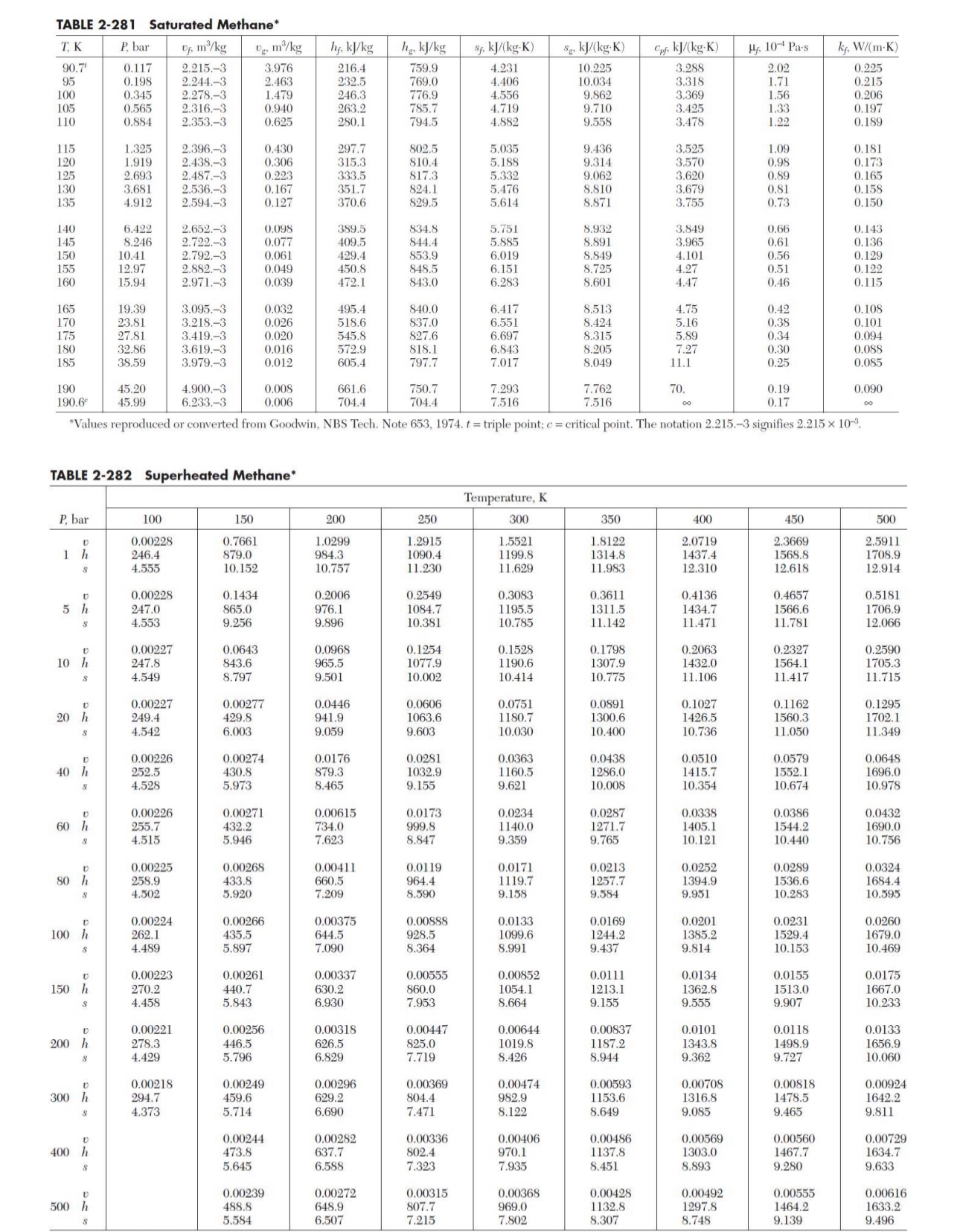
Methane is liquefied in a Claude liquefaction system as shown below. Gaseous Methane is compressed to 8 0 bar and precooled to 3 0 0
Methane is liquefied in a Claude liquefaction system as shown below. Gaseous Methane is compressed to bar and precooled to K stream This highpressure methane gas is further cooled in heat exchanger I to a temperature of K steam and then split into two streams, a drawoff stream of to the expander stream with the main stream the remaining flowing to the throttle valve via heat exchanger II streams to The expander and throttle valve exhaust to a pressure of bar streams and The temperature of the methane gas leaving the expander stream is K Recycle methane gas stream leaves the exchanger system at bar and KDetermine the following:i The specific enthalpy kJkg of streams and ii If kgh of liquid methane stream is produced, the required mass flow rate kgh of methane in streams and iii The temperature deg C and specific enthalpy of streams and iv The power kW generated by the expander and the efficiency of the expanderNote: You may assume that there is no pressure drop across the heat exchangers or the separator for this process.Properties of saturated methane at bar: Temperature K Specific enthalpy of liquid, Hf kJ kg Specific enthalpy of vapour, Hg kJ kg Specific entropy of liquid, Sf kJ kg K Specific entropy of vapour, Sg kJ kg K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


