Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Methane is oxidized with air to produce formaldehyde in a continuous reactor. A competing reaction is the combustion of methane to form CO2. CH4(g) +
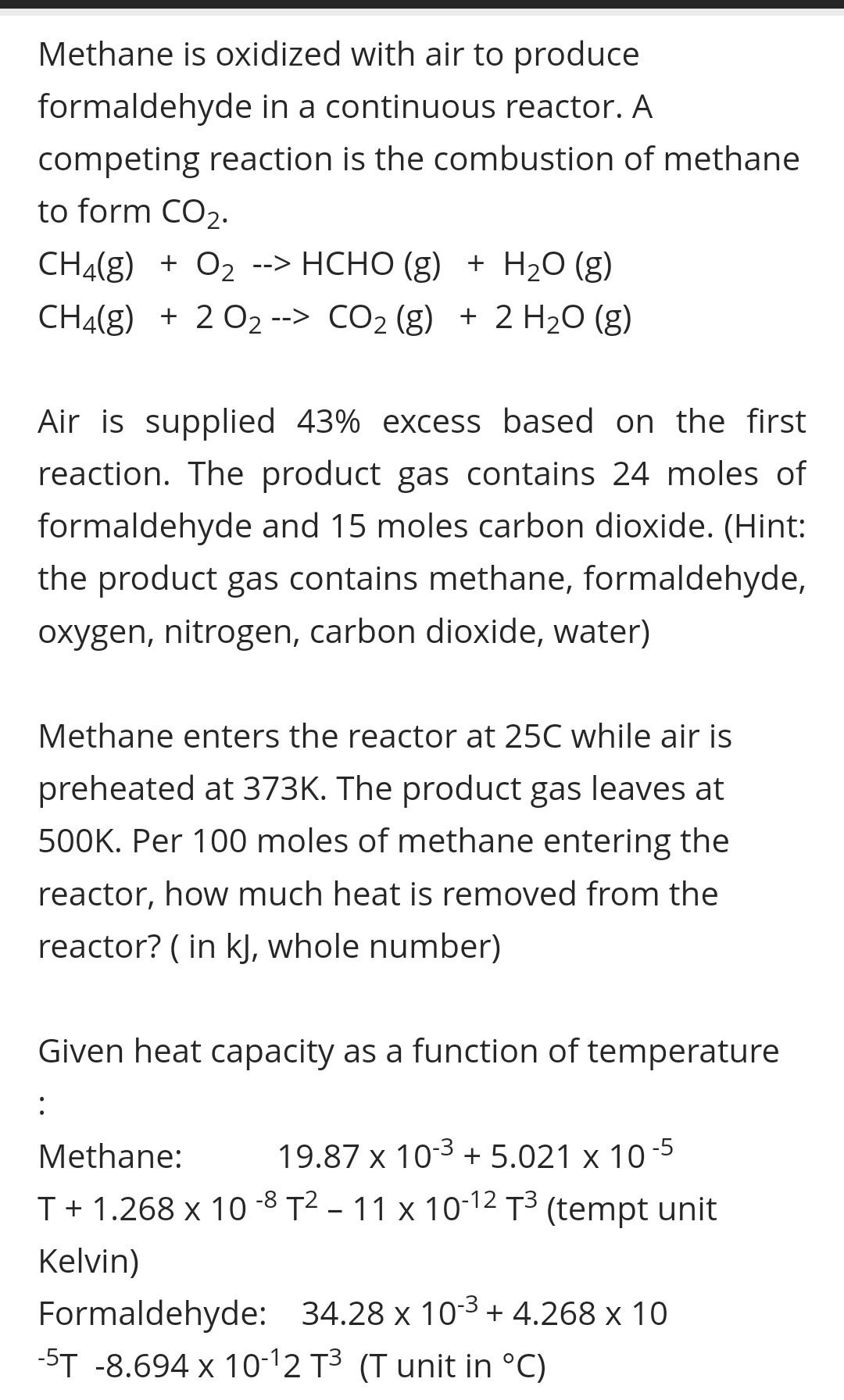
Methane is oxidized with air to produce formaldehyde in a continuous reactor. A competing reaction is the combustion of methane to form CO2. CH4(g) + O2 --> HCHO (g) + H2O (g) CH4(g) + 2 02 --> CO2 (g) + 2 H2O (g) Air is supplied 43% excess based on the first reaction. The product gas contains 24 moles of formaldehyde and 15 moles carbon dioxide. (Hint: the product gas contains methane, formaldehyde, oxygen, nitrogen, carbon dioxide, water) Methane enters the reactor at 25C while air is preheated at 373K. The product gas leaves at 500K. Per 100 moles of methane entering the reactor, how much heat is removed from the reactor? (in kJ, whole number) Given heat capacity as a function of temperature : Methane: 19.87 x 10-3 + 5.021 x 10-5 T + 1.268 x 10-8 T2 - 11 x 10-12 T3 (tempt unit Kelvin) Formaldehyde: 34.28 x 10-3+ 4.268 x 10 -5T -8.694 x 10-12 T3 (T unit in C)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


