Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Methanol is formed from carbon monoxide and hydrogen in the gas phase reaction C O + 2 H 2 C H 3 O H The
Methanol is formed from carbon monoxide and hydrogen in the gas phase reaction
The equilibrium constant at is If a reactor is fed with gas composed of mole mole CO and the balance Nan inert gas and reaction occurs at atm, determine the conversion of CO in the reactor assuming equilibrium is established. Assume a basis of moles feed.
PLEASE SEE: Please work it out correctly and send the answer written down on a paper. Thanks!
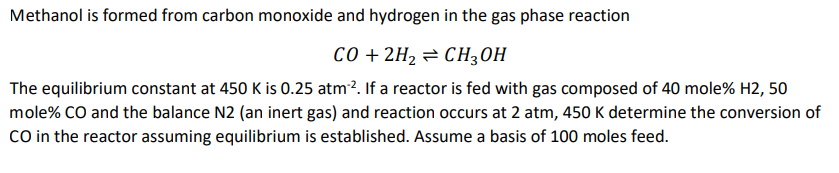
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


