Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(7) In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the e label Compound A: CH Government
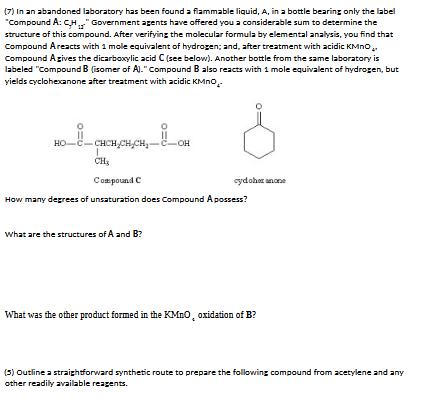

(7) In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the e label "Compound A: CH" Government agents have offered you a considerable sum to determine the structure of this compound. After verifying the molecular formula by elemental analysis, you find that Compound Areacts with 1 mole equivalent of hydrogen; and, after treatment with acidic KMnO.. Compound Agives the dicarboxylic acid C (see below). Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mole equivalent of hydrogen, but yields cyclohexanone after treatment with acidic KMnO.. HO-C-CHCH,CH,CH-C-OH CH3 Compound C cydohet anone How many degrees of unsaturation does Compound A possess? What are the structures of A and B? What was the other product formed in the KMnO, oxidation of B? (5) Outline a straightforward synthetic route to prepare the following compound from acetylene and any other readily available reagents. 3. (4) When 3,6-dimethylcyclohexene is reacted with dry gaseous HBr, one of the products is 1-bromo-1,4- dimethylcyclohexane. Provide a detailed step-by-step mechanism showing electron flow to explain the formation of this product.
Step by Step Solution
★★★★★
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


