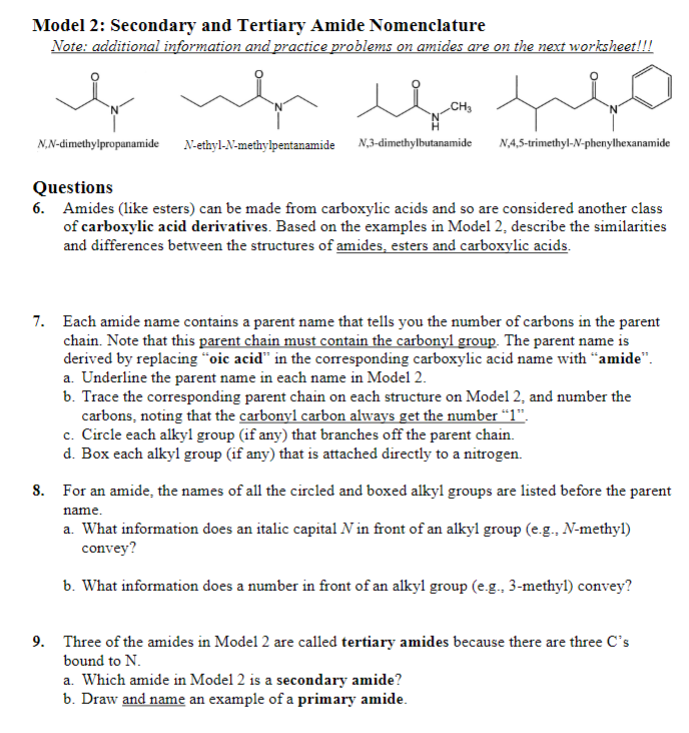
Model 2: Secondary and Tertiary Amide Nomenclature Note: additional information and practice problems on amides are on the next worksheet!!! N,N-dimethylpropanamide N-ethyl-N-methylpentanamide N,3-dimethylbutanamide N,4,5-trimethyl- N-phenylhexanamide Questions 6. Amides (like esters) can be made from carboxylic acids and so are considered another class of carboxylic acid derivatives. Based on the examples in Model 2, describe the similarities and differences between the structures of amides, esters and carboxylic acids. 7. Each amide name contains a parent name that tells you the number of carbons in the parent chain. Note that this parent chain must contain the carbonyl group. The parent name is derived by replacing "oic acid" in the corresponding carboxylic acid name with "amide". a. Underline the parent name in each name in Model 2. b. Trace the corresponding parent chain on each structure on Model 2, and number the carbons, noting that the carbonyl carbon always get the number "1". c. Circle each alkyl group (if any) that branches off the parent chain. d. Box each alkyl group (if any) that is attached directly to a nitrogen. 8. For an amide, the names of all the circled and boxed alkyl groups are listed before the parent name. a. What information does an italic capital N in front of an alkyl group (e.g., N-methyl) convey? b. What information does a number in front of an alkyl group (e.g., 3-methyl) convey? 9. Three of the amides in Model 2 are called tertiary amides because there are three C's bound to N. a. Which amide in Model 2 is a secondary amide? b. Draw and name an example of a primary amide. Model 2: Secondary and Tertiary Amide Nomenclature Note: additional information and practice problems on amides are on the next worksheet!!! N,N-dimethylpropanamide N-ethyl-N-methylpentanamide N,3-dimethylbutanamide N,4,5-trimethyl- N-phenylhexanamide Questions 6. Amides (like esters) can be made from carboxylic acids and so are considered another class of carboxylic acid derivatives. Based on the examples in Model 2, describe the similarities and differences between the structures of amides, esters and carboxylic acids. 7. Each amide name contains a parent name that tells you the number of carbons in the parent chain. Note that this parent chain must contain the carbonyl group. The parent name is derived by replacing "oic acid" in the corresponding carboxylic acid name with "amide". a. Underline the parent name in each name in Model 2. b. Trace the corresponding parent chain on each structure on Model 2, and number the carbons, noting that the carbonyl carbon always get the number "1". c. Circle each alkyl group (if any) that branches off the parent chain. d. Box each alkyl group (if any) that is attached directly to a nitrogen. 8. For an amide, the names of all the circled and boxed alkyl groups are listed before the parent name. a. What information does an italic capital N in front of an alkyl group (e.g., N-methyl) convey? b. What information does a number in front of an alkyl group (e.g., 3-methyl) convey? 9. Three of the amides in Model 2 are called tertiary amides because there are three C's bound to N. a. Which amide in Model 2 is a secondary amide? b. Draw and name an example of a primary amide







