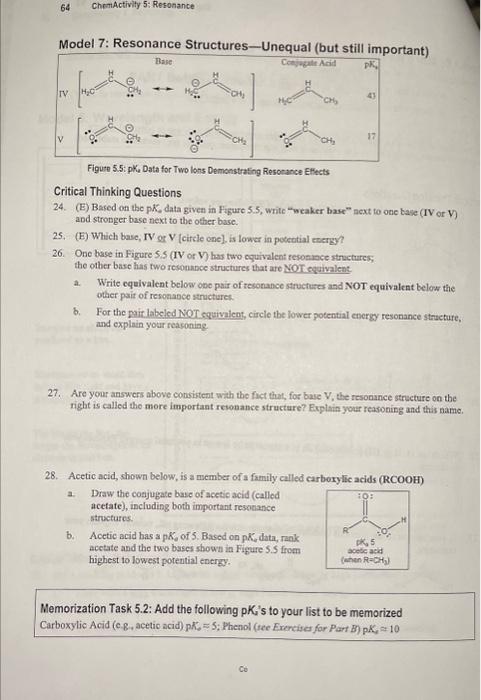
Model 7: Resonance Structures-Uneaual (but still imnortant) Figute 5.5: pKu, Data for Two lons Demonstrating Resonance Eftects Critical Thinking Questions 24. (E) Based on the RK, data given in Figure 5.5, write "weaker base" aext to one base (IV or V) and stronger base next to the other base. 25. (E) Which base, IV or V [circle one], is lower in poteutial energy? 26. One base in Figure S.S (IV or V) has two equivalent reson ince structures; the other base has two resonabce structures that are NOT equivalent. a. Write equivalent belos one pair of tesonance structures and NOT equivalent belon the other pait of resonabce stnictures. b. For the pair labeled NOT equivalens, circle the lower potential energy resonance stracture, and explain your reaconine 27. Are your answers above consistent with the fict that, for base V, the resonance structure on the right is called tho more important rebonance structure? Explain your reasoning and this name. 28. Acetic acid, shown below, is a member of a family called carborylic acids (RCOOH) a. Draw the conjugate base of acetic acid (called acetate), including both important resonance structures. b. Acetic acid has a pK,of, Sased on pKddata,rank acctate and the two bases showa in Figure S.S from highest to lowest porential energy. Memorization Task 5.2: Add the following pKd 's to your list to be memorized Carboxylic Acid (c. , acetic acid) pK5=5; Phenol (tee Exereiter for Part B ) pK410 c. Construct an explanation based on resonance arguments for your potential energy rank order in the previous question. (Note all three bases bave two important resonance structures.) d. Briefly summarize how both the quantity and quality of an ion's resonance structures affect its potential energy. Model 8: Resonance Structures of Neutral Molecules Oocasionally, you will cncounter a neutral molecule that has more than one resonance structure. There are two general types: aromatic molecules = benzene and similar molecules Use Type 3 Arrows (as deseribed for cations in Model 3) for neutral aromatic molecules. Benzene Toluene (also called Methyibentene) a common benzene dertvative Aromenle molecules are namied for the oubrs of the flat wenters of the family to be discovernd They are frequently encountered, both in noture and the labarntory, and will play a central pole in the second half of shie book) zwitterions = overall neutral molecules with both and formal charges (eig, ozone) Use Type 1 and 2 curved arrows (as described for anions in Model 3) for zwitterions Ozone Critical Thinking Questions 29. (E) Add curved arrows to the resonance structure of benzene on the left so as to generate the resonance structure of benzene on the right. What type of curved arrow(s) did you use? 30. Construct an explanation for why benzene and other aromatic molecules are low in potential energy