Question
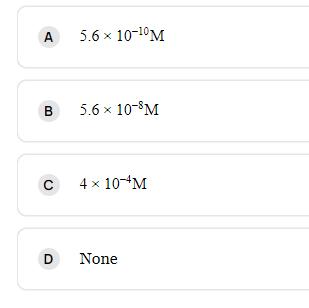
Molar solubility of Ca(OH)2 in a solution that has a pH of 12.[KSP[Ca(OH)2]=5.61012] A B C 5.6 10-0 M 5.6 10-8M 4 x 10-M D
Molar solubility of Ca(OH)2 in a solution that has a pH of 12.[KSP[Ca(OH)2]=5.6×10−12]
A B C 5.6 10-0 M 5.6 10-8M 4 x 10-M D None
Step by Step Solution
There are 3 Steps involved in it
Step: 1
So that option B is correct We know that for a solution PM POH 14 PH of the so...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Analytical Chemistry
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
9th edition
495558281, 978-0495558286
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App