Question
Multiple choice, pick the correct answer How many phosphate ions, PO4 3- are in 3.58 mol of calcium phosphate, Ca3(PO 4 ) 2 A. 6.02
Multiple choice, pick the correct answer
How many phosphate ions, PO43- are in 3.58 mol of calcium phosphate, Ca3(PO4)2
A. 6.02 X 1023
B. 4.31 x 1024
C. 2.16 x1024
D. 1.72 x 1025
E. 28.64
2. Match each letter to the correct number
A. Based on a balanced chemical reaction, the relationship between the products water and oxygen can be expressed in this way
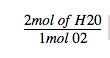
B. A piece of sodium metal is added to water it reacts violently. When the reaction is over, only a clear liquid remains in the beaker. The Sodium is an example of this.
C. A student carries out a reaction and measures the mass of the product at 1.23g
D. In the reaction of gasoline with oxygen in a car fuel tank, oxygen in a car's fuel tank, oxygen is an example of this
E. An engineering technician determines that a step in a chemical process has an efficiency of 89%
1. excess reactant
2. mole ratio
3. limiting reactant
4. actual yield
5. percentage yield
6. theoretical yield
7. experimental error
3. Your friend tells you that a decomposition reaction does not have any excess reactants do you agree or disagree? explain your answer using an example
Will give a thumbs up thank you!
2mol of H20 Imol 02
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


