*must use CAPCOST*


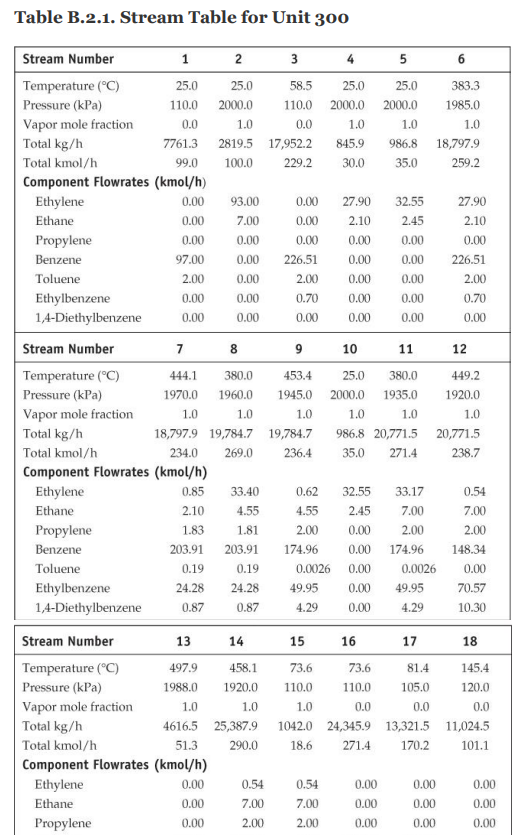
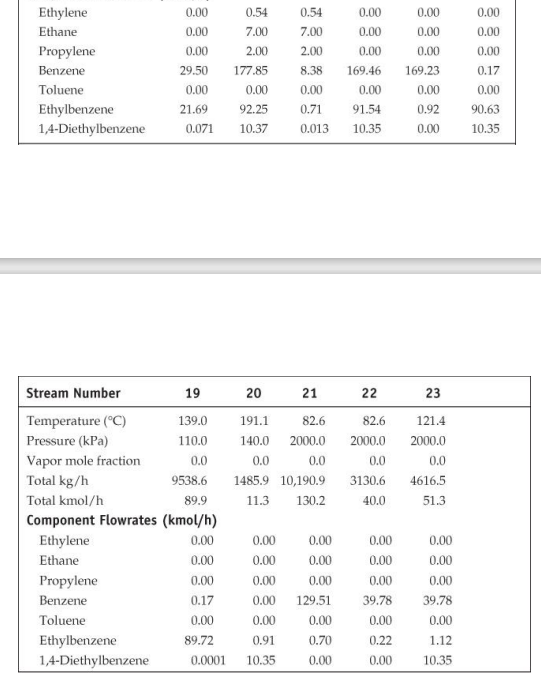
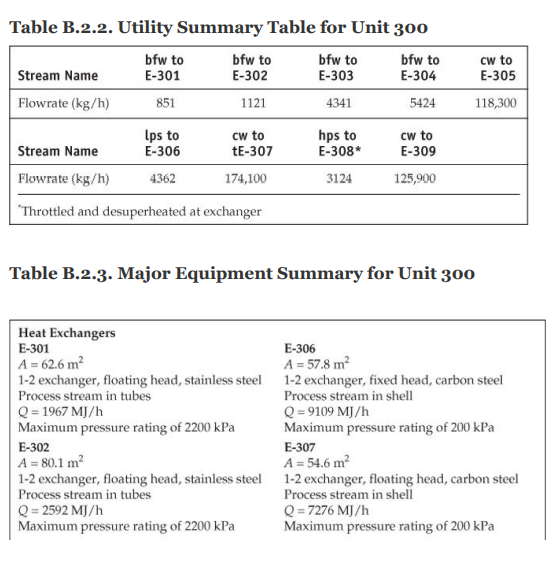
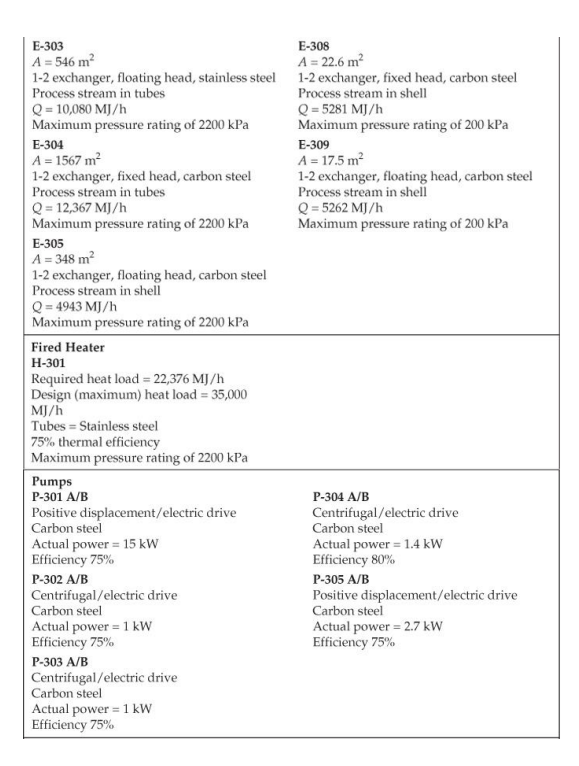
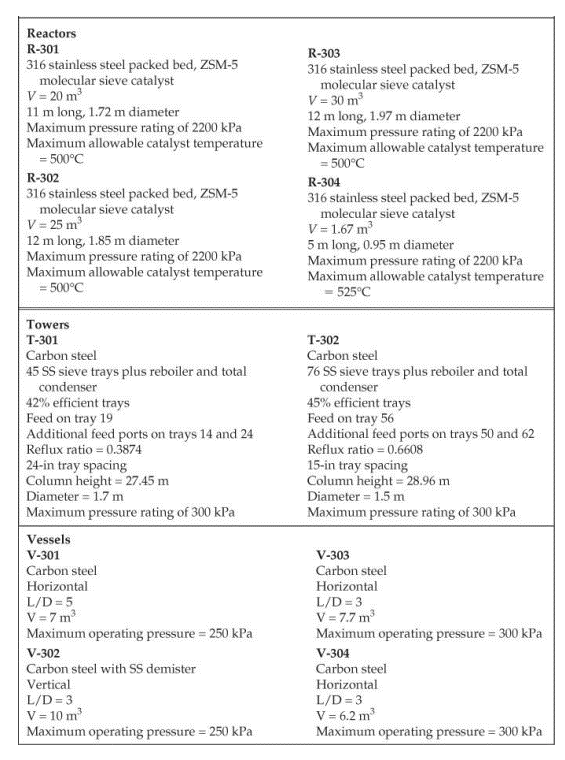

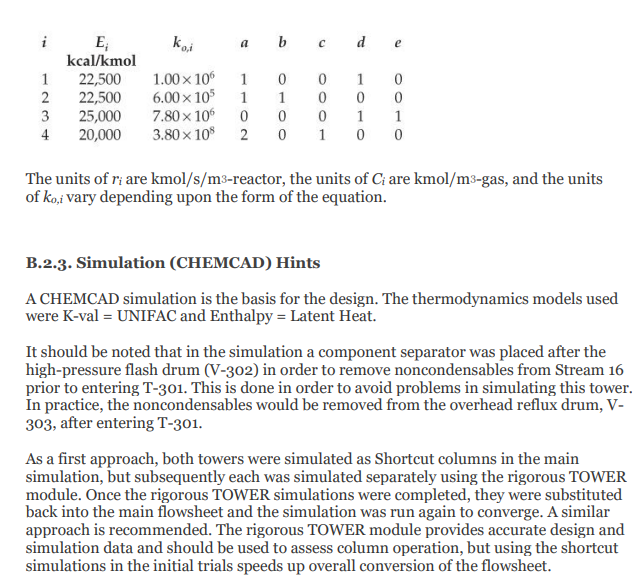
28. Cost of manufacturing (14 points) Chapter 7, problem 22 and Chapter 8, problem 14 in Turton book (see Appendix B). It is recommended that the following problems be solved using CAPCOST (use a value of CEPCI = 582). 22. Determine the bare module, total module, and grassroots cost of the ethylbenzene plant described in Appendix B, Project B.2. 14. Estimate the cost of operating labor (COL), the cost of utilities (CUT), and the cost of manufacturing (COM) for the ethylbenzene process given in Project B.2 of Appendix B. You must do Problem 7.22 in order to estimate COMd. Note: please take screen captures from CAPCOST showing your results, instead of submitting the Excel file. B.2. ETHYLBENZENE PRODUCTION, UNIT 300 The majority of ethylbenzene (EB) processes produce EB for internal consumption within a coupled process that produces styrene monomer. The facility described here produces 80,000 tonne/y of 99.8 mol% ethylbenzene that is totally consumed by an on-site styrene facility. As with most EB/styrene facilities, there is significant heat integration between the two plants. In order to decouple the operation of the two plants, the energy integration is achieved by the generation and consumption of steam within the two processes. The EB reaction is exothermic, so steam is produced, and the styrene reaction is endothermic, so energy is transferred in the form of steam. B.2.1. Process Description [1, 2] The PFD for the EB process is shown in Figure B.2.1. A refinery cut of benzene is fed from storage to an on-site process vessel (V-301), where it is mixed with the recycled benzene. From V-301, it is pumped to a reaction pressure of approximately 2000 kPa (20 atm) and sent to a fired heater (H-301) to bring it to reaction temperature (approximately 400C). The preheated benzene is mixed with feed ethylene just prior to entering the first stage of a reactor system consisting of three adiabatic packed-bed reactors (R-301 to R-303), with interstage feed addition and cooling. Reaction occurs in the gas phase and is exothermic. The hot, partially converted reactor effluent leaves the first packed bed, is mixed with more feed ethylene, and is fed to E-301, where the stream is cooled to 380C prior to passing to the second reactor (R-302), where further reaction takes place. High-pressure steam is produced in E-301, and this steam is subsequently used in the styrene unit. The effluent stream from R-302 is similarly mixed with feed ethylene and is cooled in E-302 (with generation of high-pressure steam) prior to entering the third and final packed-bed reactor, R- 303. The effluent stream leaving the reactor contains products, by-products, unreacted benzene, and small amounts of unreacted ethylene and other noncondensable gases. The reactor effluent is cooled in two waste-heat boilers (E-303 and E-304), in which high-pressure and low-pressure steam, respectively, is generated. This steam is also consumed in the styrene unit. The two-phase EB V-301 H-301 R-301/23 E-30112 R-304 E-303 E-304 E-305 V-302 T-301 E-306 E-307 V-303 P-302 NB T-302 E-308 E-309 V-304 P-303 NB Benzene Feed Ethylbenzene Reactor Trans- HP LP Reactor LN Benzene Benzene Benzone Benzone Benzone EB EB EB EB Feed Heater Reactors Inter alkylation Steam Steam Eluent Separator Tower Reboiler Condenser Reflux Reflux Tower Reboiler Condenser Reflux Reflux Drum coolers Reactor Boiler Boiler Cooler Drum Pumps Drum Pumpe P-301 AB Benzone P-304 AB P-305 AB DEB Benzene Feed Recycle Recycle Pumps Pumps Pumps Benzene 200 400 110 Fuel Gas 380 380 V-302 cw E-307 V-301 % -300) Home 110 T-301 H-301 R-30117 R 303 P-302 AB P-301 A/B FI 0. XD os By E-303 E-304 E-305 hps los P-305 AB 6-301 TE btw 80 w E300 Ethylbenzene Ethylene btw w 280 170 500 V-306 T-302 P-303 AB R-304 kPa E-308 2000 hips H-301 P-304 A/B Figure B.2.1. Unit 300: Ethylbenzene Process Flow Diagram 0.54 0.00 0.54 7.00 2.00 0.00 0.00 7.00 0.00 2.00 0.00 0.00 Ethylene Ethane Propylene Benzene Toluene Ethylbenzene 1,4-Diethylbenzene 0.00 0.00 0.00 29.50 0.00 21.69 0.071 177.85 0.17 0.00 0.00 0.00 169.23 0.00 0.92 0.00 8.38 0.00 0.71 0.013 0.00 92.25 10.37 169.46 0.00 91.54 10.35 0.00 90.63 10.35 20 21 22 23 121.4 2000.0 0.0 191.1 82.6 140.0 2000.0 0.0 0.0 1485.9 10,190.9 11.3 130.2 82.6 2000.0 0.0 3130.6 40.0 0.0 4616,5 51.3 Stream Number 19 Temperature (C) 139.0 Pressure (kPa) 110.0 Vapor mole fraction Total kg/h 9538.6 Total kmol/h 89.9 Component Flowrates (kmol/h) Ethylene 0.00 Ethane 0.00 Propylene 0.00 Benzene 0.17 Toluene 0.00 Ethylbenzene 89.72 1,4-Diethylbenzene 0.0001 0.00 0.00 0.00 0.00 0.00 0.91 10.35 0.00 0.00 0.00 129.51 0.00 0.70 0.00 0.00 0.00 0.00 39.78 0.00 0.22 0.00 0.00 0.00 0.00 39.78 0.00 1.12 10.35 cw to E-305 118,300 Table B.2.2. Utility Summary Table for Unit 300 bfw to bfw to bfw to bfw to Stream Name E-301 E-302 E-303 E-304 Flowrate (kg/h) 851 1121 4341 5424 lps to hps to cw to Stream Name E-306 TE-307 E-308 E-309 Flowrate (kg/h) 4362 174,100 3124 125,900 "Throttled and desuperheated at exchanger cw to Table B.2.3. Major Equipment Summary for Unit 300 Heat Exchangers E-301 E-306 A = 62.6 m2 A = 57.8 m 1-2 exchanger, floating head, stainless steel 1-2 exchanger, fixed head, carbon steel Process stream in tubes Process stream in shell Q = 1967 MJ/h Q = 9109 MJ/h Maximum pressure rating of 2200 kPa Maximum pressure rating of 200 kPa E-302 E-307 A = 80.1 m2 A = 54.6 m2 1-2 exchanger, floating head, stainless steel 1-2 exchanger, floating head, carbon steel Process stream in tubes Process stream in shell Q = 2592 MJ/h Q = 7276 MJ/h Maximum pressure rating of 2200 kPa Maximum pressure rating of 200 kPa B.2.2. Reaction Kinetics The production of EB takes place via the direct addition reaction between ethylene and benzene: CH, + CH,CH,CH, (B.2.1) benzene ethylene ethylbenzene The reaction between EB and ethylene to produce DEB also takes place: (B.2.2) C6H5CH3 + CH2CH(CH3)2 ethylbenzene ethylene diethylbenzene Additional reactions between DEB and ethylene yielding triethylbenzene (and higher) are also possible. However, in order to minimize these additional reactions, the molar ratio of benzene to ethylene is kept high, at approximately 8:1. The production of DEB is undesirable, and its value as a side product is low. In addition, even small amounts of DEB in EB cause significant processing problems in the downstream styrene process. Therefore, the maximum amount of DEB in EB is specified as 2 ppm. In order to maximize the production of the desired EB, the DEB is separated and returned to a separate reactor in which excess benzene is added to produce EB via the following equilibrium reaction: CH(CH3)2 + CH = 2CH.CH (B.2.3) diethylbenzene benzene ethylbenzene The incoming benzene contains a small amount of toluene impurity. The toluene reacts with ethylene to form ethyl benzene and propylene: (B.2.4) CH-CH3 + 2C,H, CHCH3 + C3H, toluene ethylbenzene propylene The reaction kinetics derived for a new catalyst are given as ->;=k, e-Ei/RTO "Cenylene C&B Cioluene benzene CDEB (B.2.5) where i is the reaction number above (B.2.1), and the following relationships pertain

















