Answered step by step
Verified Expert Solution
Question
1 Approved Answer
N H 3 - air mixture containing 5 % N H 3 is scrubbed with water to remove N H 3 , 5 0 0
air mixture containing is scrubbed with water to remove of
gas mixture is to be processed with of water to reduce in the exit gas to
Calculate the height of packing required assuming dilute solutions are involved.
Equilibrium relation is given by where mole fraction of in liquid and
vapor. HTU
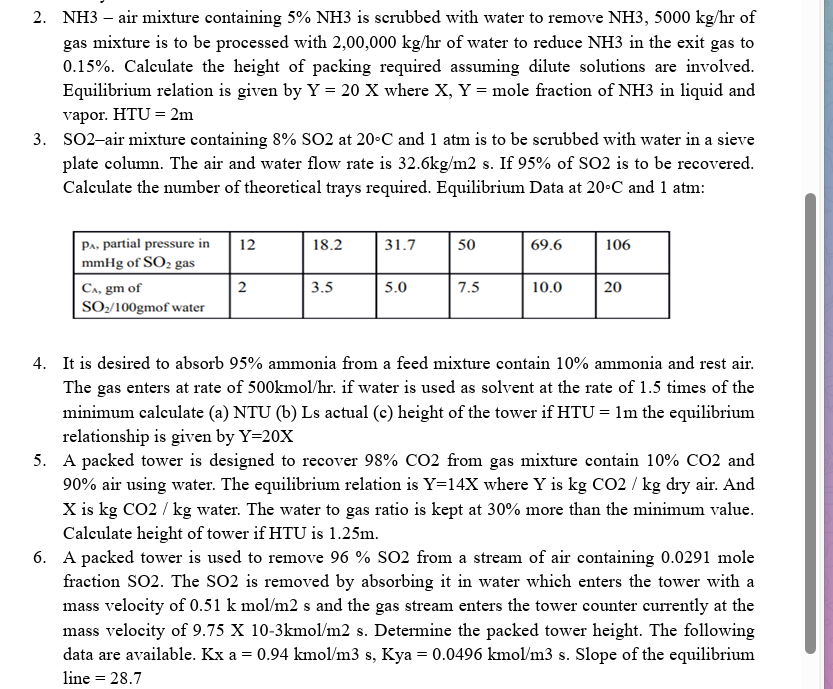
SOair mixture containing at and atm is to be scrubbed with water in a sieve
plate column. The air and water flow rate is s If of is to be recovered.
Calculate the number of theoretical trays required. Equilibrium Data at and atm :
It is desired to absorb ammonia from a feed mixture contain ammonia and rest air.
The gas enters at rate of kmo if water is used as solvent at the rate of times of the
minimum calculate a NTU b Ls actual c height of the tower if HTU the equilibrium
relationship is given by
A packed tower is designed to recover from gas mixture contain and
air using water. The equilibrium relation is where is kgCO dry air. And
is kgCO water. The water to gas ratio is kept at more than the minimum value.
Calculate height of tower if HTU is
A packed tower is used to remove from a stream of air containing mole
fraction The is removed by absorbing it in water which enters the tower with a
mass velocity of kmo and the gas stream enters the tower counter currently at the
mass velocity of kmo Determine the packed tower height. The following
data are available. KxakmoKyakmo Slope of the equilibrium
line
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


