Answered step by step
Verified Expert Solution
Question
1 Approved Answer
N2(g)+3F2(g) +2NF3(g) AH,98= -426 kJ mol! The following questions relate to the synthesis reaction represented by the chemical equation in the box above. a. Calculate
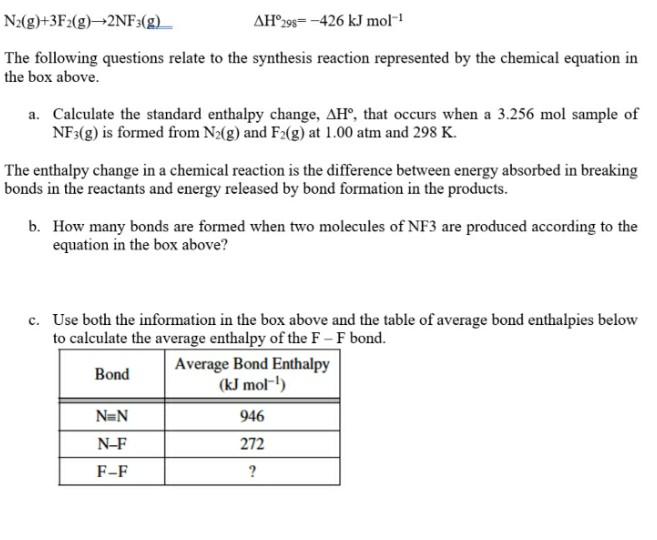
N2(g)+3F2(g) +2NF3(g) AH,98= -426 kJ mol! The following questions relate to the synthesis reaction represented by the chemical equation in the box above. a. Calculate the standard enthalpy change, AH, that occurs when a 3.256 mol sample of NF3(g) is formed from N2(g) and F2(g) at 1.00 atm and 298 K. The enthalpy change in a chemical reaction is the difference between energy absorbed in breaking bonds in the reactants and energy released by bond formation in the products. b. How many bonds are formed when two molecules of NF3 are produced according to the equation in the box above? c. Use both the information in the box above and the table of average bond enthalpies below to calculate the average enthalpy of the F - F bond. Bond Average Bond Enthalpy (kJ mol!) NEN 946 N-F 272 F-F
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


