Answered step by step
Verified Expert Solution
Question
1 Approved Answer
N2(g)+3H2(g)NH3(g)+ energy Imagine a cylinder containing nitrogen gas (N2), hydrogen gas (H2), and ammonia gas (NH3). The chemicals in the cylinder are in a state
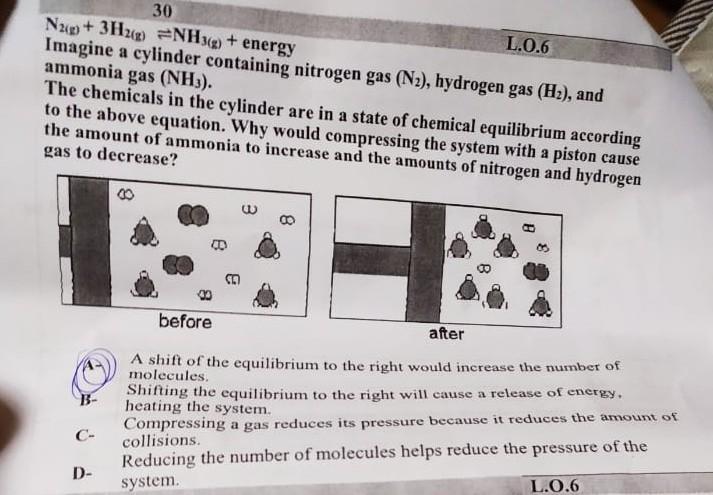
N2(g)+3H2(g)NH3(g)+ energy Imagine a cylinder containing nitrogen gas (N2), hydrogen gas (H2), and ammonia gas (NH3). The chemicals in the cylinder are in a state of chemical equilibrium according to the above equation. Why would compressing the system with a piston cause the amount of ammonia to increase and the amounts of nitrooen and b. irogen gas to decrease? (A-) A shift of the equilibrium to the right would increase the number of B- Shifting the equilibrium to the right will cause a release of energy, C- Compressing a gas reduces its pressure because it reduces the amount of collisions. collisions. D- Reducing the number of molecules helps reduce the pressure of the system
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


