Answered step by step
Verified Expert Solution
Question
1 Approved Answer
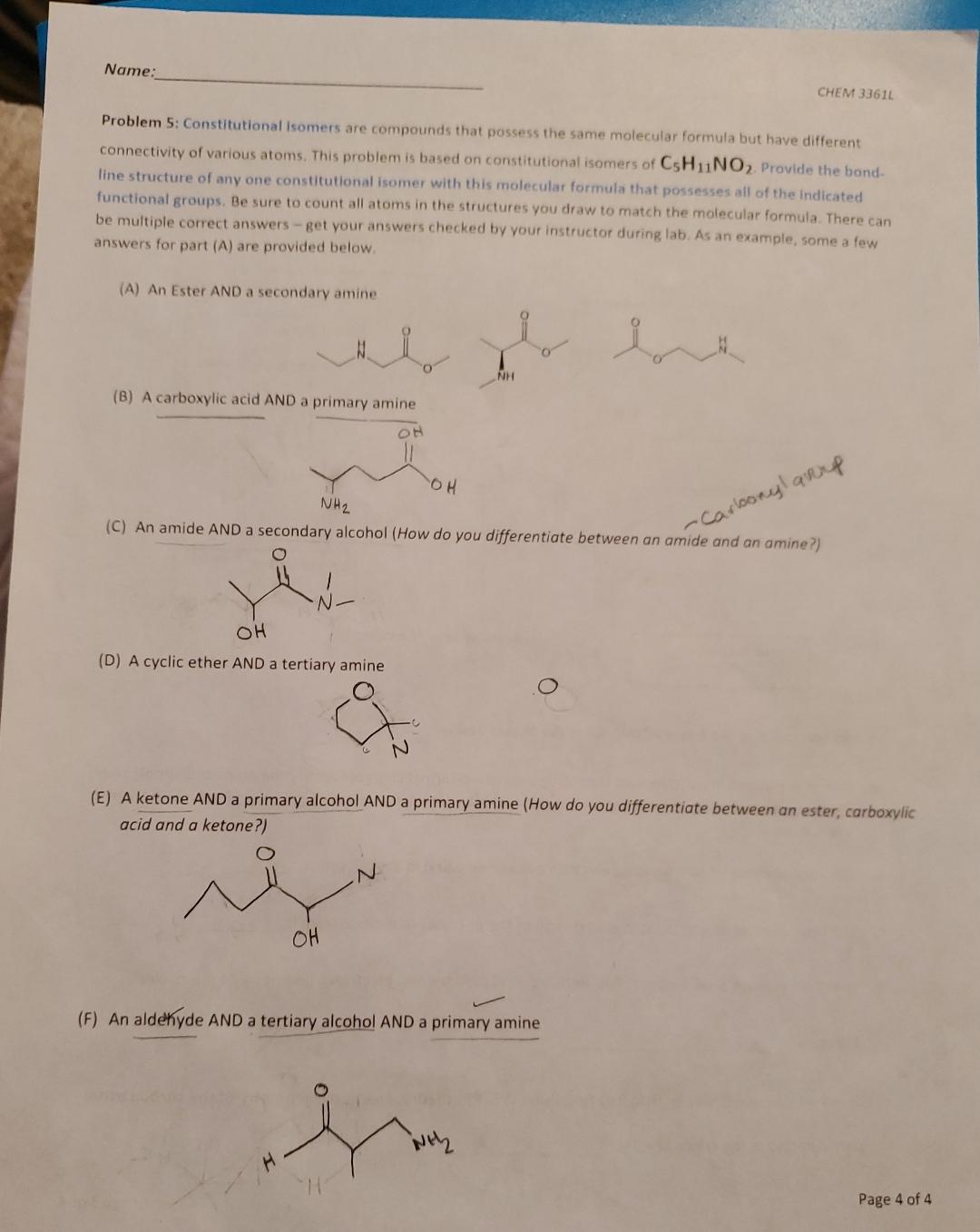
Nam CHEM 3 3 6 1 4 Problem 5 : Constitutional isomers are compounds that possess the same molecular formula but have different connectivity of
Nam
CHEM
Problem : Constitutional isomers are compounds that possess the same molecular formula but have different connectivity of various atoms. This problem is based on constitutional isomers of Provide the bond. line structure of any one constitutional isomer with this molecular formula that possesses all of the indicated functional groups. Be sure to count all atoms in the structures you draw to match the molecular formula. There can be multiple correct answers get your answers checked by your instructor during lab. As an example, some a few answers for part A are provided below.
A An Ester ANI
B A carboxylic a
C An amide AND a secondary alcohol How do you differentiate between an amide and an amine?
D A cyclic ether AND a tertiarv amine
E A ketone AND a primary alcohol AND a primary amine How do you differentiate between an ester, carboxylic acid and a ketone?
F An aldeyde AND a tertiary alcohol AND a primary amine
Page of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


