Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Name: Acrylic acid is produced by the catalytic partial oxidation of propylene in the presence of steam at elevated temperature and ambient pressure. In
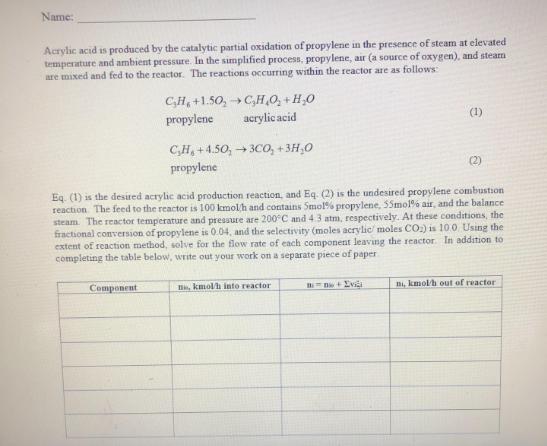
Name: Acrylic acid is produced by the catalytic partial oxidation of propylene in the presence of steam at elevated temperature and ambient pressure. In the simplified process, propylene, air (a source of oxygen), and steam are mixed and fed to the reactor. The reactions occurring within the reactor are as follows C,H, +1.50, C,H,O, + H,0 propylene acrylic acid (1) C,H, + 4.50, 3C0, + 3H,0 propylene (2) Eq. (1) is the desired acrylic acid production reaction, and Eq. (2) is the undesired propylene combustion reaction The feed to the reactor is 100 kmolh and contains Smol% propylene, S5mol% air, and the balance steam. The reactor temperature and pressure are 200C and 4.3 atm, respectively. At these conditions, the fractional conversion of propylene is 0.04, and the selectivity (moles acrylic moles CO.) is 10.0 Using the extent of reaction method, solve for the flow rate of each component leaving the reactor. In addition to completing the table below, write out your work on a separate piece of paper Component , kmolh into reactor ni, kmol/h out of reactor
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Given the total feed100 Kmol Given C 3 H 6 5mol5Kmol Given Air55mol55Kmol Given O 2 550211155Kmol Gi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


