Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need ASAP Q1 0.1 kg of air is compressed polytropically with a polytropic exponent of 1.3 from 102 kPa and 20 C to 500 kPa.
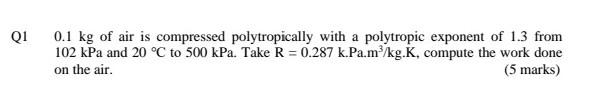

need ASAP
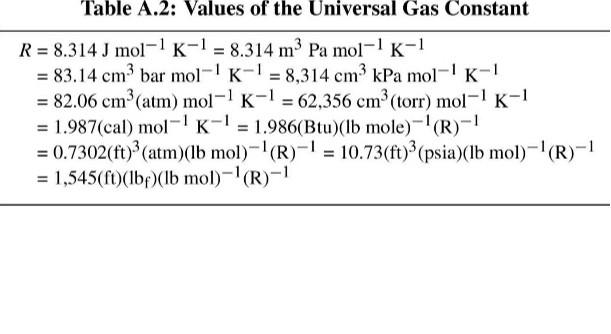
Q1 0.1 kg of air is compressed polytropically with a polytropic exponent of 1.3 from 102 kPa and 20 C to 500 kPa. Take R = 0.287 k.Pa.m/kg.K, compute the work done on the air (5 marks) Quantity Length Mass Force Pressure Volume Table A.1: Conversion Factors Conversion I m= 100 cm = 3.28084(ft) = 39.3701(in) 1 kg = 103 g = 2.20462(Ibm) IN=1 kg m-2 = 10%(dyne) = 0.224809(lb) 1 bar = 10 kg m-1,-2 = 10 Nm-2 = 10 Pa = 10 kPa = 10%(dyne) cm-2 = 0.986923(atm) = 14.5038(psia) = 750.061(tor) I m = 10 cm = 103 liters = 35.3147(ft)? = 264.172(gal) 1 g em? = 10kg m-3 = 62.4278(Ibm Xf)-3 1J = 1 kg ms-2 = 1 Nm = 1 m Pa = 10-m? bar = 10 cm bar = 9.86923 cm (atm) = 10% (dyne) cm = 10'erg) =0.239006(cal) = 5.12197 10-(f(psia) = 0.737562(ft)clbr) =9.47831 x 10- (Btu) = 2.77778 x10-7 kWhr 1 kW = 103 W = 10 kg m?s-3 = 102JS = 239.006(cal)-1 = 737.562(ft)(lbf) s - =0.947831(Btu)-1 = 1.34102(hp) Density Energy Power = Table A.2: Values of the Universal Gas Constant R = 8.314 J mol-1 K-1 = 8.314 m3 Pa mol-1 K-1 = 83.14 cm bar mol-' K-1 = 8,314 cm3 kPa mol-1 K-1 = 82.06 cm (atm) mol-1 K-1 = 62,356 cm (torr) mol-1 K-1 = 1.987(cal) mol-' K-1 = 1.986(Btu)(Ib mole)-'(R)-! = 0.7302(ft)2 (atm)(Ib mol)-'(R)-1 = 10.73(ft)(psia)(Ib mol)-'(R)-1 = 1,545(ft)(be)(b mol)-'(R)-1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


