Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help 4. Using the average of the data from your three measurements of the pH of a saturated solution of Mg(OH)2, calculate the concentration
need help 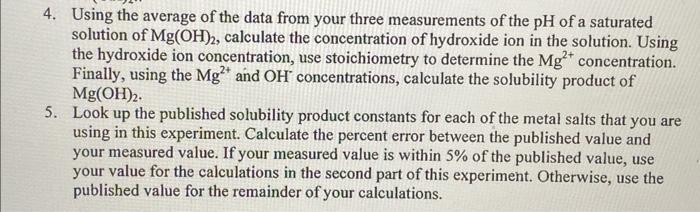
4. Using the average of the data from your three measurements of the pH of a saturated solution of Mg(OH)2, calculate the concentration of hydroxide ion in the solution. Using the hydroxide ion concentration, use stoichiometry to determine the Mg2+ concentration. Finally, using the Mg2+ and OH2 concentrations, calculate the solubility product of Mg(OH)2. 5. Look up the published solubility product constants for each of the metal salts that you are using in this experiment. Calculate the percent error between the published value and your measured value. If your measured value is within 5% of the published value, use your value for the calculations in the second part of this experiment. Otherwise, use the published value for the remainder of your calculations. 4. Using the average of the data from your three measurements of the pH of a saturated solution of Mg(OH)2, calculate the concentration of hydroxide ion in the solution. Using the hydroxide ion concentration, use stoichiometry to determine the Mg2+ concentration. Finally, using the Mg2+ and OH2 concentrations, calculate the solubility product of Mg(OH)2. 5. Look up the published solubility product constants for each of the metal salts that you are using in this experiment. Calculate the percent error between the published value and your measured value. If your measured value is within 5% of the published value, use your value for the calculations in the second part of this experiment. Otherwise, use the published value for the remainder of your calculations 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


