Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help answering this Q asap. Thank you. MISSED THIS? Read Section 14.6 (Pages 601-613) ; Watch KCV 14.6 IWE 14.9. Calculate the treezing point
Need help answering this Q asap. Thank you. 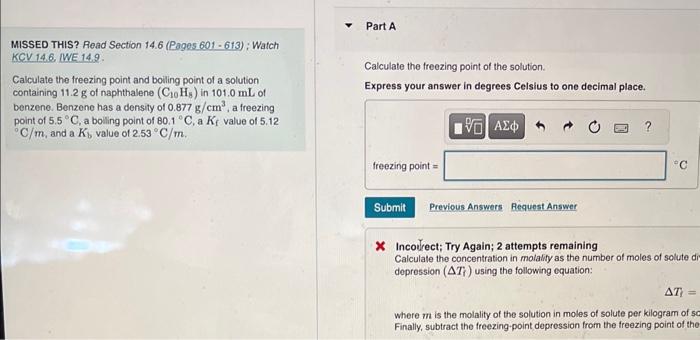
MISSED THIS? Read Section 14.6 (Pages 601-613) ; Watch KCV 14.6 IWE 14.9. Calculate the treezing point of the solution. Calculate the treezing point and bolling point of a solution containing 11.2g of naphthalene (C10Hs) in 101.0mL of benzene. Benzene has a density of 0.877g/cm3, a freezing point of 5.5C, a bolling point of 80.1C, a Kf value of 5.12 C/m, and a Kb value of 2.53C/m. x Incovrect; Try Again; 2 attempts remaining Calculate the concentration in molanly as the number of moles of solute di depression (Ti) using the following equation: T= where m is the molality of the solution in moles of solute per kilogram of 5c Finally, subtract the freezing-point depression from the freezing point of the 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


