Answered step by step
Verified Expert Solution
Question
1 Approved Answer
NEED HELP ASAP!! Give the relationship between the following pairs of structures: CH2=CHCH2Cl and CHCl=CHCH3 stereoisomers same compound not isomers constitutional isomers Predict the hybridization
NEED HELP ASAP!! 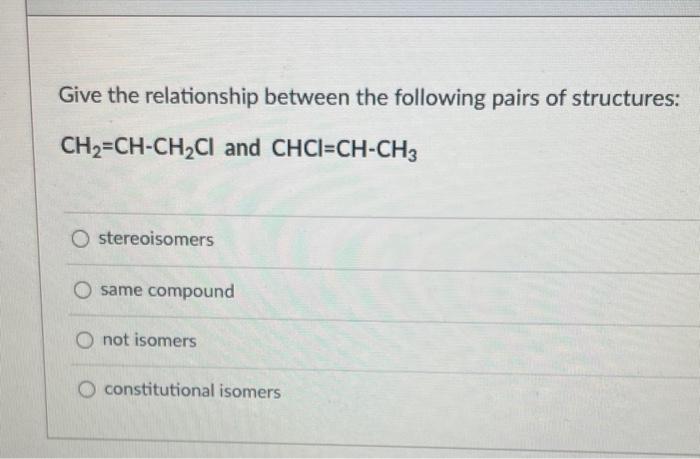
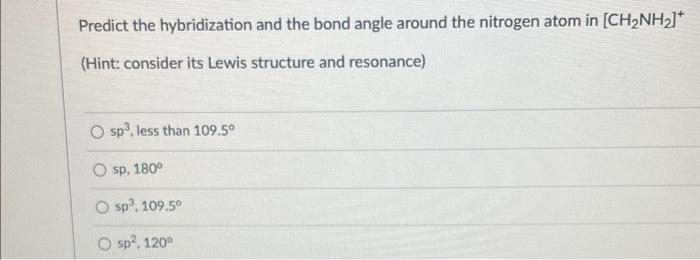
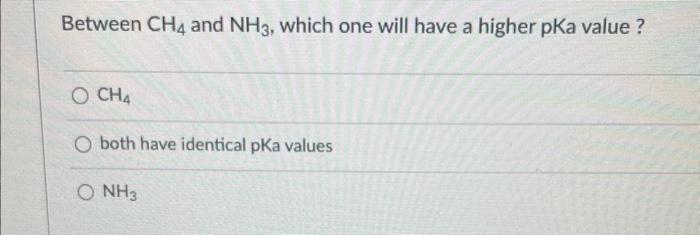
Give the relationship between the following pairs of structures: CH2=CHCH2Cl and CHCl=CHCH3 stereoisomers same compound not isomers constitutional isomers Predict the hybridization and the bond angle around the nitrogen atom in [CH2NH2]+ (Hint: consider its Lewis structure and resonance) sp3, less than 109.5 sp, 180 5p3,109.5 5p2,120 Between CH4 and NH3, which one will have a higher pKa value? CH4 both have identical pKa values NH3 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


