Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help calculating the molar mass of the unknown compound based on the freezing point depression for all three unknowns trials. 7. Calculate the molar
need help calculating the molar mass of the unknown compound based on the freezing point depression for all three unknowns trials. 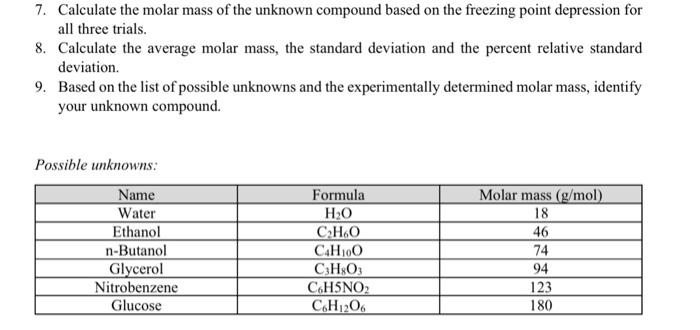
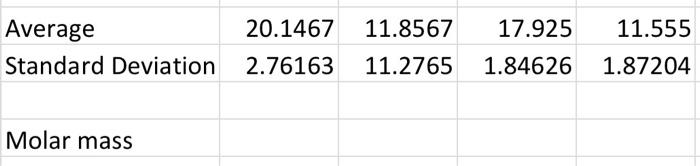
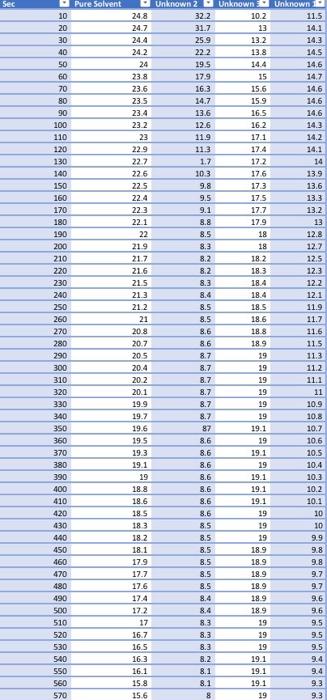
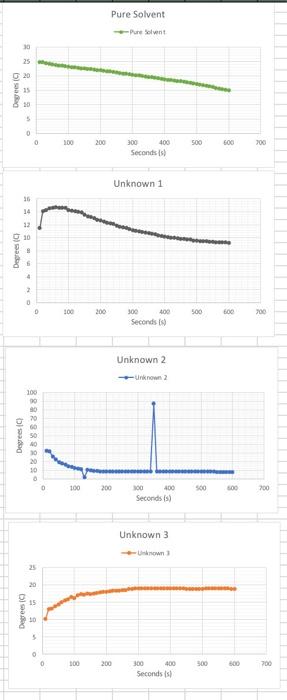
7. Calculate the molar mass of the unknown compound based on the freezing point depression for all three trials. 8. Calculate the average molar mass, the standard deviation and the percent relative standard deviation. 9. Based on the list of possible unknowns and the experimentally determined molar mass, identify your unknown compound. Possible unknowns: Name Water Ethanol n-Butanol Glycerol Nitrobenzene Glucose Formula HO CHO C4H100 C:H:03 CHSNO CH,206 Molar mass (g/mol) 18 46 74 94 123 180 20.1467 11.8567 Average Standard Deviation 17.925 1.84626 11.555 1.87204 2.76163 11.2765 Molar mass Sec Pure Solvent 248 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250 260 270 280 290 300 310 320 330 340 350 360 370 380 390 400 410 420 430 440 450 460 470 480 490 500 510 520 530 540 550 560 570 24.7 24.4 242 24 23.8 23.6 23.5 23.4 23.2 23 229 22.7 226 225 22.4 223 22.1 22 219 21.7 21.6 215 21.3 212 21 20.8 20.7 205 20.4 20.2 20.1 19.9 19.7 19.6 Unknown 2 Unknown Unknown 322 10.2 11.5 31.7 13 14.1 25.9 13.2 14.3 222 13.8 14.5 19.5 14.4 14.6 17.9 15 147 16.3 15.6 14.6 14.7 15.9 14.6 13.6 16.5 14.6 12.6 16.2 14.3 11.9 17.1 14.2 11.3 17.4 14.1 1.7 17.2 14 10.3 176 139 9.8 17.3 13.6 9.5 17.5 13.3 9.1 17.7 13.2 8.8 17.9 13 8.5 18 12.8 8.3 18 12.7 8.2 182 12.5 8.2 18.3 12.3 8.3 18.4 12.2 8.4 18.4 12.1 8.5 18.5 11.9 8.5 18.6 11.7 8.6 188 11.6 8.6 18.9 11.5 8.7 19 11.3 8.7 19 11.2 8.7 19 11.1 8.7 19 11 8.7 19 10.9 8.7 19 10.8 87 19.1 10.7 8.6 19 10.6 8.6 19.1 10.5 8.6 19 10.4 8.6 19.1 10.3 8.6 19.1 10.2 8.6 19.1 10.1 8.6 19 10 8.5 19 10 8.5 19 9.9 8.5 18.9 9.8 8.5 18.9 9.8 8.5 18.9 9.7 8.5 18.9 9.7 8.4 189 9.6 8.4 189 9.6 8.3 19 9.5 8.3 19 9.5 8.3 19 9.5 8.2 19.1 9.4 8.1 19.1 9.4 8.1 191 9.3 8 19 9.3 195 RE 19.3 19.1 19 188 18.6 18.5 183 18.2 18.1 17.9 17.7 17.6 17.4 17.2 17 16.7 16.5 163 16.1 15.8 15.6 Pure Solvent Sant 30 25 20 35 Degrees 30 | 5 0 0 100 200 500 600 200 300 400 Seconds) Unknown 1 36 14 32 10 Degrees (o 4 2 D 100 200 500 600 700 300 400 Seconds to Unknown 2 200 Degrees C) 883 238889 100 200 500 00 200 OD 400 Seconds is) Unknown 3 nown 25 9 15 Degrees 5 0 300 200 300 500 700 300 400 Seconds is 7. Calculate the molar mass of the unknown compound based on the freezing point depression for all three trials. 8. Calculate the average molar mass, the standard deviation and the percent relative standard deviation. 9. Based on the list of possible unknowns and the experimentally determined molar mass, identify your unknown compound. Possible unknowns: Name Water Ethanol n-Butanol Glycerol Nitrobenzene Glucose Formula HO CHO C4H100 C:H:03 CHSNO CH,206 Molar mass (g/mol) 18 46 74 94 123 180 20.1467 11.8567 Average Standard Deviation 17.925 1.84626 11.555 1.87204 2.76163 11.2765 Molar mass Sec Pure Solvent 248 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250 260 270 280 290 300 310 320 330 340 350 360 370 380 390 400 410 420 430 440 450 460 470 480 490 500 510 520 530 540 550 560 570 24.7 24.4 242 24 23.8 23.6 23.5 23.4 23.2 23 229 22.7 226 225 22.4 223 22.1 22 219 21.7 21.6 215 21.3 212 21 20.8 20.7 205 20.4 20.2 20.1 19.9 19.7 19.6 Unknown 2 Unknown Unknown 322 10.2 11.5 31.7 13 14.1 25.9 13.2 14.3 222 13.8 14.5 19.5 14.4 14.6 17.9 15 147 16.3 15.6 14.6 14.7 15.9 14.6 13.6 16.5 14.6 12.6 16.2 14.3 11.9 17.1 14.2 11.3 17.4 14.1 1.7 17.2 14 10.3 176 139 9.8 17.3 13.6 9.5 17.5 13.3 9.1 17.7 13.2 8.8 17.9 13 8.5 18 12.8 8.3 18 12.7 8.2 182 12.5 8.2 18.3 12.3 8.3 18.4 12.2 8.4 18.4 12.1 8.5 18.5 11.9 8.5 18.6 11.7 8.6 188 11.6 8.6 18.9 11.5 8.7 19 11.3 8.7 19 11.2 8.7 19 11.1 8.7 19 11 8.7 19 10.9 8.7 19 10.8 87 19.1 10.7 8.6 19 10.6 8.6 19.1 10.5 8.6 19 10.4 8.6 19.1 10.3 8.6 19.1 10.2 8.6 19.1 10.1 8.6 19 10 8.5 19 10 8.5 19 9.9 8.5 18.9 9.8 8.5 18.9 9.8 8.5 18.9 9.7 8.5 18.9 9.7 8.4 189 9.6 8.4 189 9.6 8.3 19 9.5 8.3 19 9.5 8.3 19 9.5 8.2 19.1 9.4 8.1 19.1 9.4 8.1 191 9.3 8 19 9.3 195 RE 19.3 19.1 19 188 18.6 18.5 183 18.2 18.1 17.9 17.7 17.6 17.4 17.2 17 16.7 16.5 163 16.1 15.8 15.6 Pure Solvent Sant 30 25 20 35 Degrees 30 | 5 0 0 100 200 500 600 200 300 400 Seconds) Unknown 1 36 14 32 10 Degrees (o 4 2 D 100 200 500 600 700 300 400 Seconds to Unknown 2 200 Degrees C) 883 238889 100 200 500 00 200 OD 400 Seconds is) Unknown 3 nown 25 9 15 Degrees 5 0 300 200 300 500 700 300 400 Seconds is 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


