Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help calculating yellow values! Due soon. Specific heat is 18-8 stainless steel. :: Calorimeter Lab Data Saving.. File Edit View Insert Format Data Tools
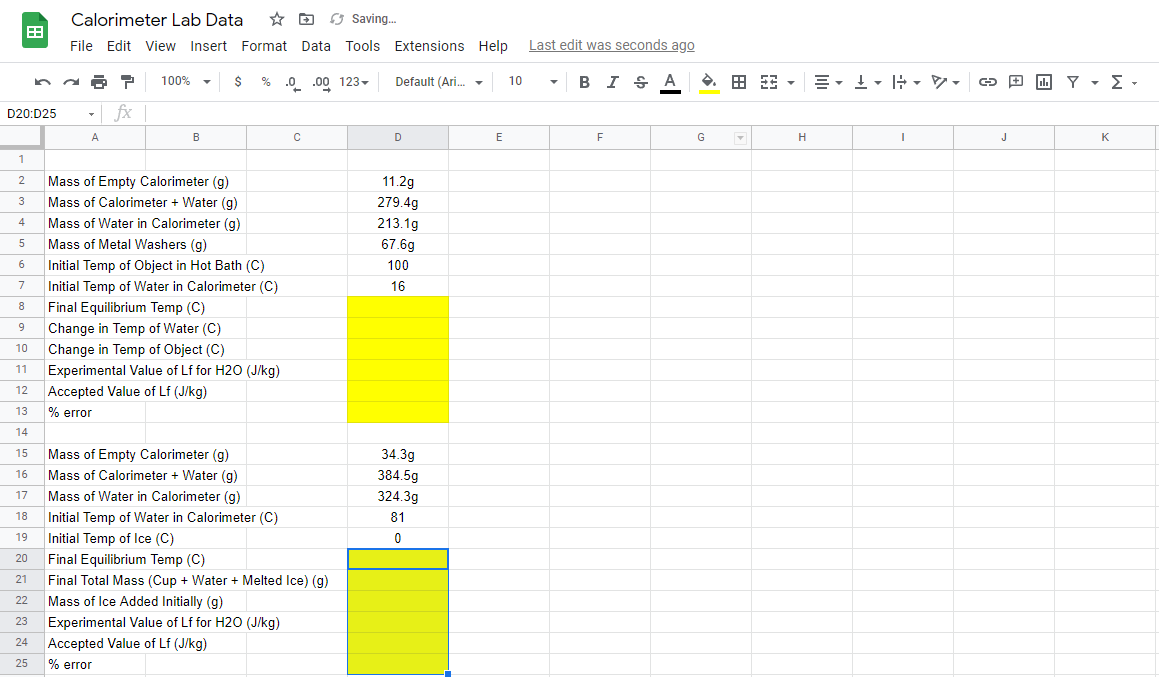 Need help calculating yellow values! Due soon.
Need help calculating yellow values! Due soon.
Specific heat is 18-8 stainless steel.
:: Calorimeter Lab Data Saving.. File Edit View Insert Format Data Tools Extensions Help Last edit was seconds ago A 100% $ % 0.00123 Default (Ari... - 10 BI. A 3 - II. - , D20:025 - fx A B D E F G H 1 J K 1 2 3 4 11.2g 279.49 213.1g 67.69 100 16 5 6 7 Mass of Empty Calorimeter (9) Mass of Calorimeter + Water (g) Mass of Water in Calorimeter (9) Mass of Metal Washers (g) Initial Temp of Object in Hot Bath (C) Initial Temp of Water in Calorimeter (C) Final Equilibrium Temp (C) Change in Temp of Water (C) Change in Temp of Object (C) Experimental Value of Lf for H2O (J/kg) Accepted Value of Lf (J/kg) % error 8 9 10 11 12 13 14 15 16 17 34.3g 384.5g 324.3g 81 0 18 19 20 Mass of Empty Calorimeter (9) Mass of Calorimeter + Water (9) Mass of Water in Calorimeter (9) Initial Temp of Water in Calorimeter (C) Initial Temp of Ice (C) Final Equilibrium Temp (C) Final Total Mass (Cup + Water + Melted Ice) (9) Mass of Ice Added Initially (g) Experimental Value of Lf for H2O (J/kg) Accepted Value of Lf (J/kg) % error 21 22 23 24 25 :: Calorimeter Lab Data Saving.. File Edit View Insert Format Data Tools Extensions Help Last edit was seconds ago A 100% $ % 0.00123 Default (Ari... - 10 BI. A 3 - II. - , D20:025 - fx A B D E F G H 1 J K 1 2 3 4 11.2g 279.49 213.1g 67.69 100 16 5 6 7 Mass of Empty Calorimeter (9) Mass of Calorimeter + Water (g) Mass of Water in Calorimeter (9) Mass of Metal Washers (g) Initial Temp of Object in Hot Bath (C) Initial Temp of Water in Calorimeter (C) Final Equilibrium Temp (C) Change in Temp of Water (C) Change in Temp of Object (C) Experimental Value of Lf for H2O (J/kg) Accepted Value of Lf (J/kg) % error 8 9 10 11 12 13 14 15 16 17 34.3g 384.5g 324.3g 81 0 18 19 20 Mass of Empty Calorimeter (9) Mass of Calorimeter + Water (9) Mass of Water in Calorimeter (9) Initial Temp of Water in Calorimeter (C) Initial Temp of Ice (C) Final Equilibrium Temp (C) Final Total Mass (Cup + Water + Melted Ice) (9) Mass of Ice Added Initially (g) Experimental Value of Lf for H2O (J/kg) Accepted Value of Lf (J/kg) % error 21 22 23 24 25Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


