Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help filling in these blanks: First blank either 1 or 2 second blank either back-side attack or a carbocation intermediate third blank either water
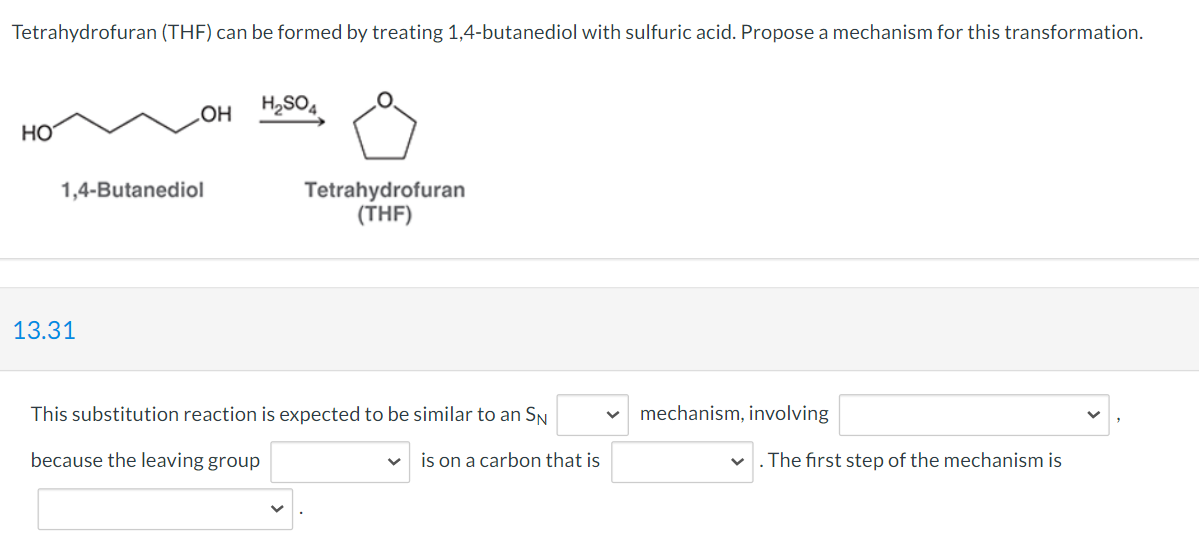
need help filling in these blanks:
First blank either "1" or "2"
second blank either "back-side attack" or "a carbocation intermediate"
third blank either "water" or "hydroxide"
4th blank either "tertiary," "secondary," or "primary"
5th blank either "nucleophilic attack," "loss of a leaving group," "protonation of the diol," or "deprotonation of the diol"
Tetrahydrofuran (THF) can be formed by treating 1,4-butanediol with sulfuric acid. Propose a mechanism for this transformation. OH H2SO4 HO 1,4-Butanediol Tetrahydrofuran (THF) 13.31 This substitution reaction is expected to be similar to an SN mechanism, involving because the leaving group is on a carbon that is V The first step of the mechanism isStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


