Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help filling out chart assuming solution A has a initial concentration of 0.02 M KIO3 and Solution B has an initial concentration 0.002 M
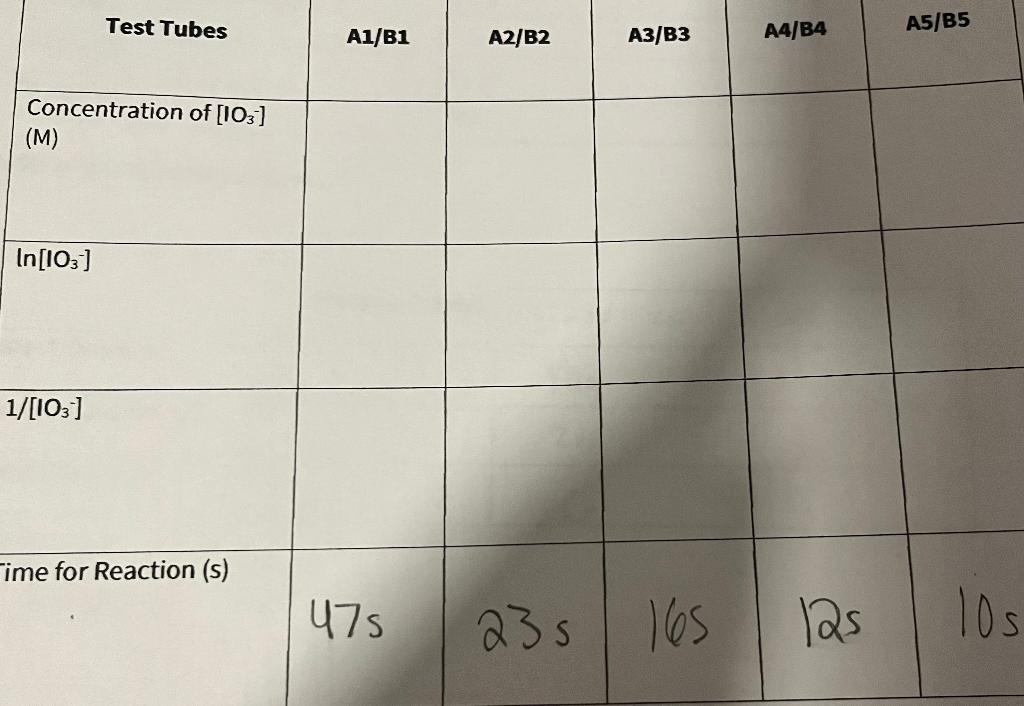
Need help filling out chart assuming solution A has a initial concentration of 0.02 M KIO3 and Solution B has an initial concentration 0.002 M NaHSO3.
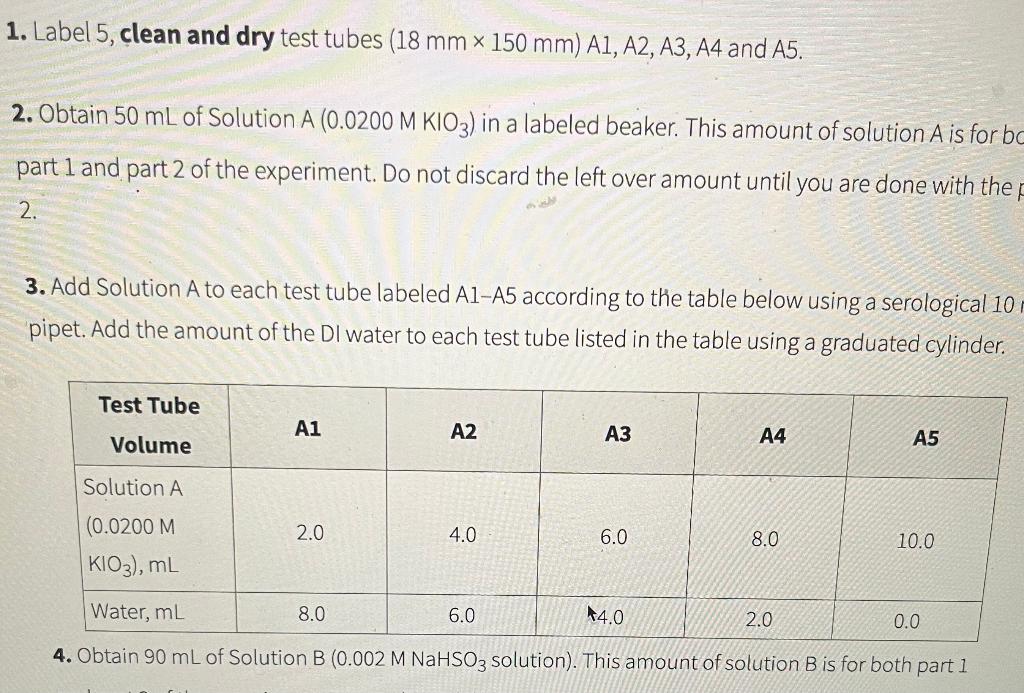
1. Label 5 , clean and dry test tubes (18mm150mm)A1,A2,A3,A4 and A5. 2. Obtain 50mL of Solution A(0.0200MKIO3) in a labeled beaker. This amount of solution A is for bo part 1 and part 2 of the experiment. Do not discard the left over amount until you are done with the 2. 3. Add Solution A to each test tube labeled A1-A5 according to the table below using a serological 10 pipet. Add the amount of the DI water to each test tube listed in the table using a graduated cylinder. 4. Obtain 90mL of Solution B(0.002MNaHSO3 solution). This amount of solution B is for bothStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


