Question
Need help in answering question after creating graph from data table and need help determining number of moles of a strong acid that must be
Need help in answering question after creating graph from data table and need help determining number of moles of a strong acid that must be added to 25 mL of the buffer to change the pH of the solution by one pH unit. calculate the average number of moles of strong acid that must be added to 25 mL of the buffer to change pH of the solution by one pH unit
What happens to the average number of moles of strong acid that must be added when the [base]/[acid] ratio decreases? Why does the average number of moles of strong acid change as it does?
Thank you
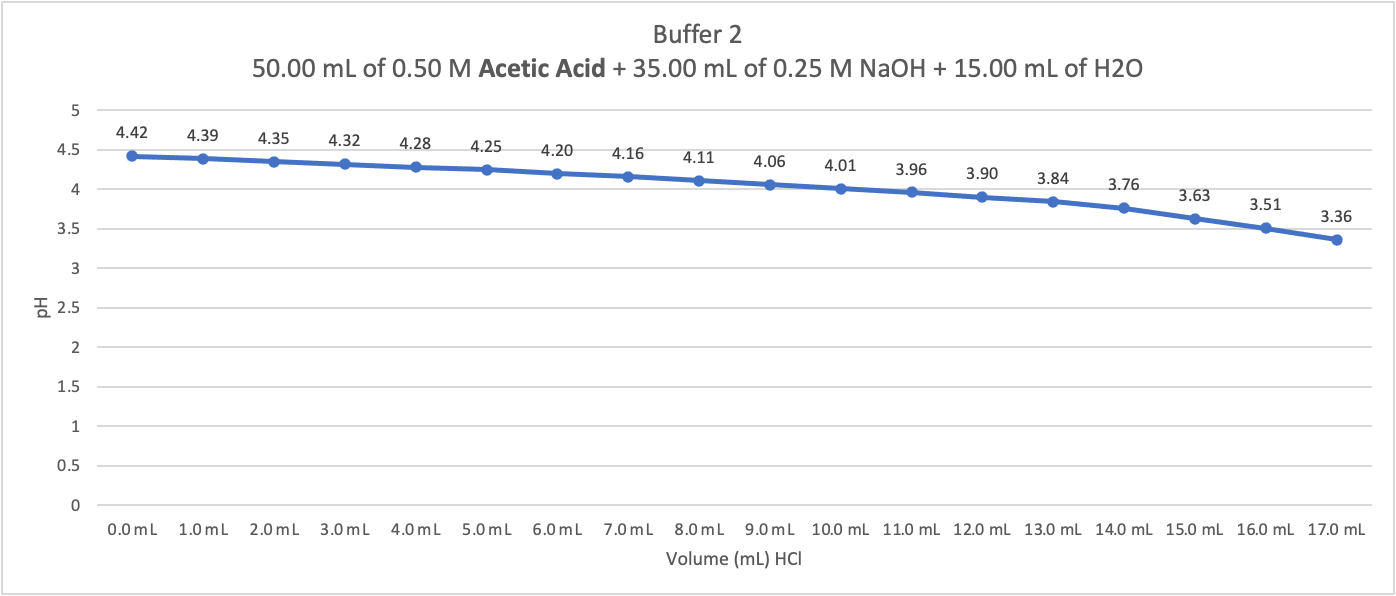 Buffer 2 50.00mL of 0.50M Acetic Acid +35.00mL of 0.25MNaOH+15.00mL of H2O 2.5 2 1.5 1 0.5 0 Volume(mL)HCl Buffer 2 50.00mL of 0.50M Acetic Acid +35.00mL of 0.25MNaOH+15.00mL of H2O 2.5 2 1.5 1 0.5 0 Volume(mL)HCl
Buffer 2 50.00mL of 0.50M Acetic Acid +35.00mL of 0.25MNaOH+15.00mL of H2O 2.5 2 1.5 1 0.5 0 Volume(mL)HCl Buffer 2 50.00mL of 0.50M Acetic Acid +35.00mL of 0.25MNaOH+15.00mL of H2O 2.5 2 1.5 1 0.5 0 Volume(mL)HCl Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


