Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help Part II: Determination of the Effect of Common Ions on the pH of Hydroxide Solutions Before you proceed with the data collection in
need help 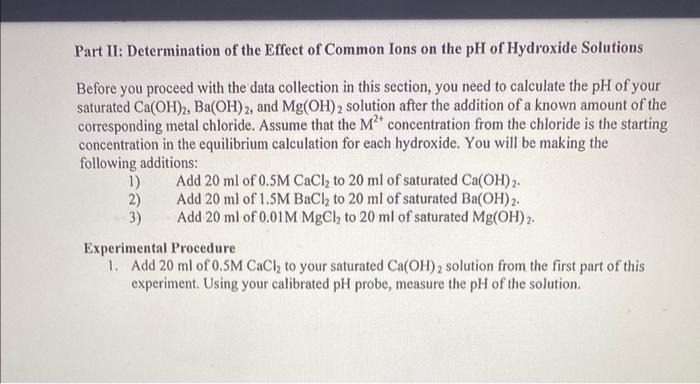
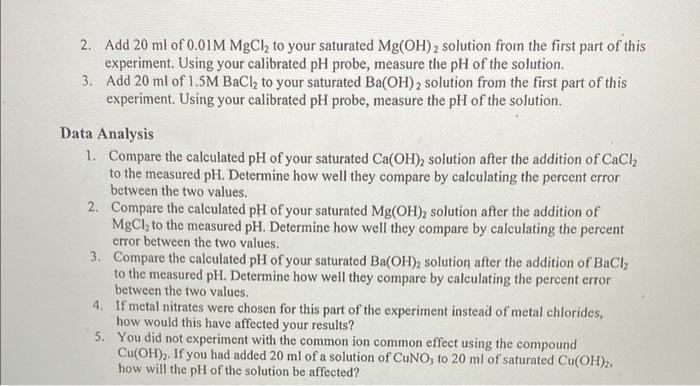
Part II: Determination of the Effect of Common Ions on the pH of Hydroxide Solutions Before you proceed with the data collection in this section, you need to calculate the pH of your saturated Ca(OH)2,Ba(OH)2, and Mg(OH)2 solution after the addition of a known amount of the corresponding metal chloride. Assume that the M2+ concentration from the chloride is the starting concentration in the equilibrium calculation for each hydroxide. You will be making the following additions: 1) Add 20ml of 0.5MCaCl2 to 20ml of saturated Ca(OH)2. 2) Add 20ml of 1.5MBaCl2 to 20ml of saturated Ba(OH)2. 3) Add 20ml of 0.01MMgCl2 to 20ml of saturated Mg(OH)2. Experimental Procedure 1. Add 20ml of 0.5MCaCl2 to your saturated Ca(OH)2 solution from the first part of this experiment. Using your calibrated pH probe, measure the pH of the solution. 2. Add 20ml of 0.01MMgCl2 to your saturated Mg(OH)2 solution from the first part of this experiment. Using your calibrated pH probe, measure the pH of the solution. 3. Add 20ml of 1.5MBaCl2 to your saturated Ba(OH)2 solution from the first part of this experiment. Using your calibrated pH probe, measure the pH of the solution. Data Analysis 1. Compare the calculated pH of your saturated Ca(OH)2 solution after the addition of CaCl2 to the measured pH. Determine how well they compare by calculating the percent error between the two values. 2. Compare the calculated pH of your saturated Mg(OH)2 solution after the addition of MgCl2 to the measured pH. Determine how well they compare by calculating the percent error between the two values. 3. Compare the calculated pH of your saturated Ba(OH)2 solution after the addition of BaCl2 to the measured pH. Determine how well they compare by calculating the percent error between the two values. 4. If metal nitrates were chosen for this part of the experiment instead of metal chlorides, how would this have affected your results? 5. You did not experiment with the common ion common effect using the compound Cu(OH)2. If you had added 20ml of a solution of CuNO3 to 20ml of saturated Cu(OH)2, how will the pH of the solution be affected 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


