Answered step by step
Verified Expert Solution
Question
1 Approved Answer
NEED HELP. Please show your work. I'll give thumbs up. Thanks For the calculations of the intial eates the concentrations are as follows: HSO3- =
NEED HELP. Please show your work. I'll give thumbs up. Thanks
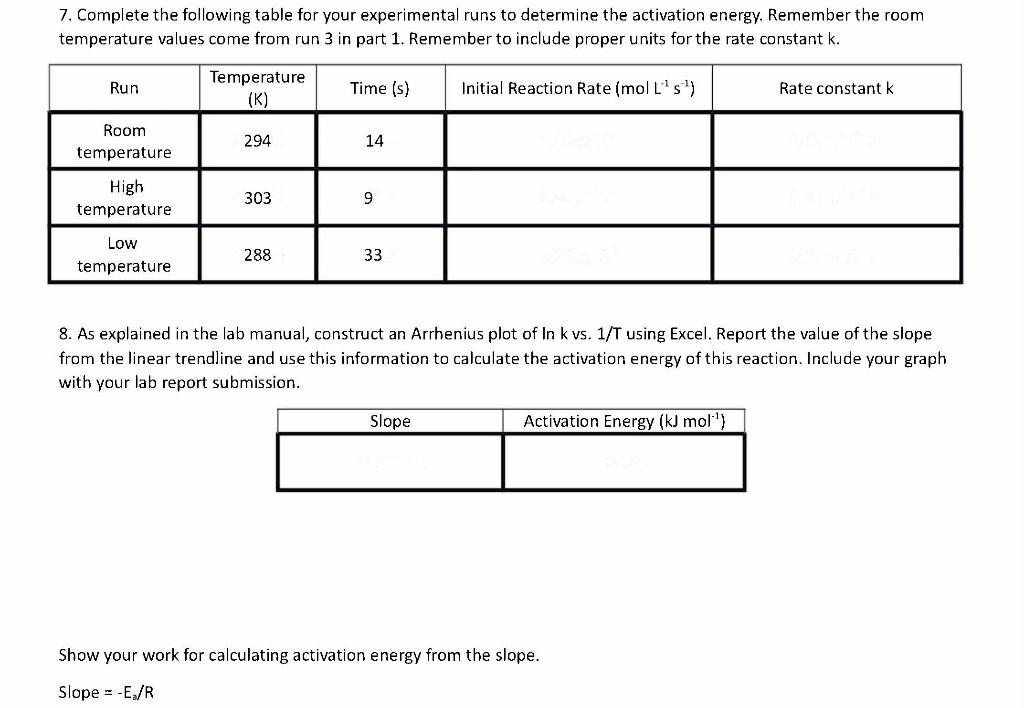
For the calculations of the intial eates the concentrations are as follows:
7. Complete the following table for your experimental runs to determine the activation energy. Remember the room temperature values come from run 3 in part 1 . Remember to include proper units for the rate constant k. 8. As explained in the lab manual, construct an Arrhenius plot of In kvs.1/T using Excel. Report the value of the slope from the linear trendline and use this information to calculate the activation energy of this reaction. Include your graph with your lab report submission. Show your work for calculating activation energy from the slope. Slope =Ea/R HSO3- = 0.001 M L-1
IO3- = 0.0003 M L-1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


