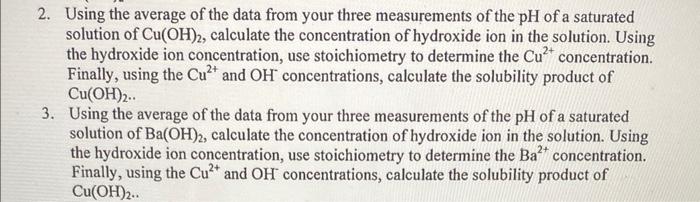
2. Using the average of the data from your three measurements of the pH of a saturated solution of Cu(OH)2, calculate the concentration of hydroxide ion in the solution. Using the hydroxide ion concentration, use stoichiometry to determine the Cu2+ concentration. Finally, using the Cu2+ and OHconcentrations, calculate the solubility product of Cu(OH)2. 3. Using the average of the data from your three measurements of the pH of a saturated solution of Ba(OH)2, calculate the concentration of hydroxide ion in the solution. Using the hydroxide ion concentration, use stoichiometry to determine the Ba2+ concentration. Finally, using the Cu2+ and OHconcentrations, calculate the solubility product of Cu(OH)2. Part I: Determination of the Ksp for Cu(OH)2,Ca(OH)2,Mg(OH)2, and Ba(OH)2 Experimental Procedure 1. Pour 20ml of saturated Cu(OH)2 solution into a 50ml beaker. 2. Obtain and assemble a Vernier LabPro unit with a pH probe. You may use either a TI83+ calculator or a laptop to control your LabPro unit. 3. Calibrate the pH probe using the pH7 buffer and a pH 10 buffer as your two reference points. The calibration procedure is slightly different for groups that use a TI 83+ and groups that use a laptop computer to control the LabPro. See your instructor if you need assistance with the calibration. 4. Using the calibrated pH probe, record the pH of the saturated Cu(OH)2 solution. 5. Rinse the pH probe with distilled water, use a Kimwipe to gently wipe off the drop of distilled water that remains on the tip of the probe, and repeat the pH measurement a second time. 6. Rinse the pH probe with distilled water, use a Kimwipe to gently wipe off the drop of distilled water that remains on the tip of the probe, and repeat the pH measurement a third time. Your final pH will be recorded as the average of your three pH measurements. Do not discard the Cu(OH)2 solution as you will need it for the next part of the experiment. 7. Repeat this procedure using saturated Ca(OH)2,Mg(OH)2 and Ba(OH, solutione 2. Using the average of the data from your three measurements of the pH of a saturated solution of Cu(OH)2, calculate the concentration of hydroxide ion in the solution. Using the hydroxide ion concentration, use stoichiometry to determine the Cu2+ concentration. Finally, using the Cu2+ and OHconcentrations, calculate the solubility product of Cu(OH)2. 3. Using the average of the data from your three measurements of the pH of a saturated solution of Ba(OH)2, calculate the concentration of hydroxide ion in the solution. Using the hydroxide ion concentration, use stoichiometry to determine the Ba2+ concentration. Finally, using the Cu2+ and OHconcentrations, calculate the solubility product of Cu(OH)2. Part I: Determination of the Ksp for Cu(OH)2,Ca(OH)2,Mg(OH)2, and Ba(OH)2 Experimental Procedure 1. Pour 20ml of saturated Cu(OH)2 solution into a 50ml beaker. 2. Obtain and assemble a Vernier LabPro unit with a pH probe. You may use either a TI83+ calculator or a laptop to control your LabPro unit. 3. Calibrate the pH probe using the pH7 buffer and a pH 10 buffer as your two reference points. The calibration procedure is slightly different for groups that use a TI 83+ and groups that use a laptop computer to control the LabPro. See your instructor if you need assistance with the calibration. 4. Using the calibrated pH probe, record the pH of the saturated Cu(OH)2 solution. 5. Rinse the pH probe with distilled water, use a Kimwipe to gently wipe off the drop of distilled water that remains on the tip of the probe, and repeat the pH measurement a second time. 6. Rinse the pH probe with distilled water, use a Kimwipe to gently wipe off the drop of distilled water that remains on the tip of the probe, and repeat the pH measurement a third time. Your final pH will be recorded as the average of your three pH measurements. Do not discard the Cu(OH)2 solution as you will need it for the next part of the experiment. 7. Repeat this procedure using saturated Ca(OH)2,Mg(OH)2 and Ba(OH, solutione