Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help solving this one! I know the answers should be delta H = 2.5*10^6 and delta T = 30 but idk how?? Exercise #7
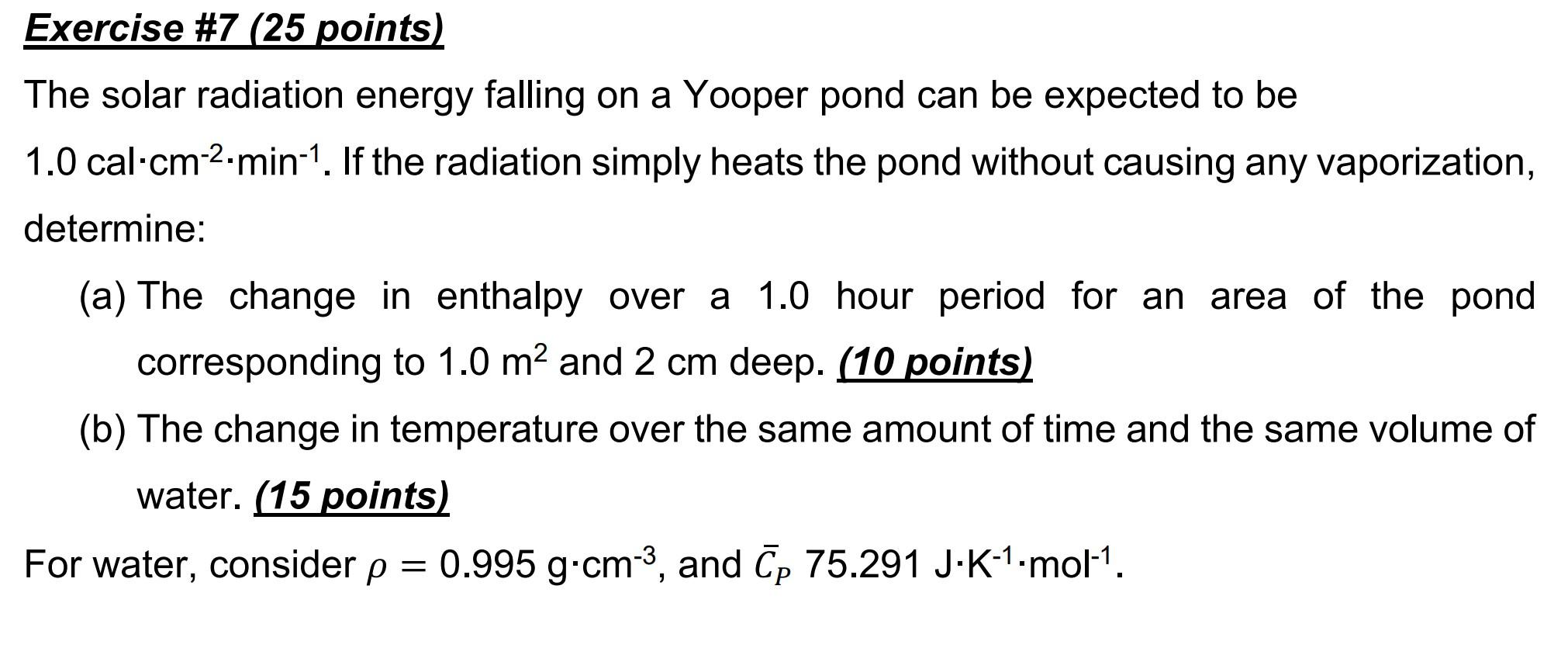
Need help solving this one! I know the answers should be delta H = 2.5*10^6 and delta T = 30 but idk how??
Exercise \#7 (25 points) The solar radiation energy falling on a Yooper pond can be expected to be 1.0calcm2min1. If the radiation simply heats the pond without causing any vaporization, determine: (a) The change in enthalpy over a 1.0 hour period for an area of the pond corresponding to 1.0m2 and 2cm deep. (10 points) (b) The change in temperature over the same amount of time and the same volume of water. (15 points) For water, consider =0.995gcm3, and CP75.291JK1mol1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


