Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need help with 7,8,9,10 Consider the following reaction and the data from 3 experiments performed at constant temperature: NH4+(aq)+NO2(aq)2H2O(l)+N2(g) 5. How is the reaction rate
Need help with 7,8,9,10 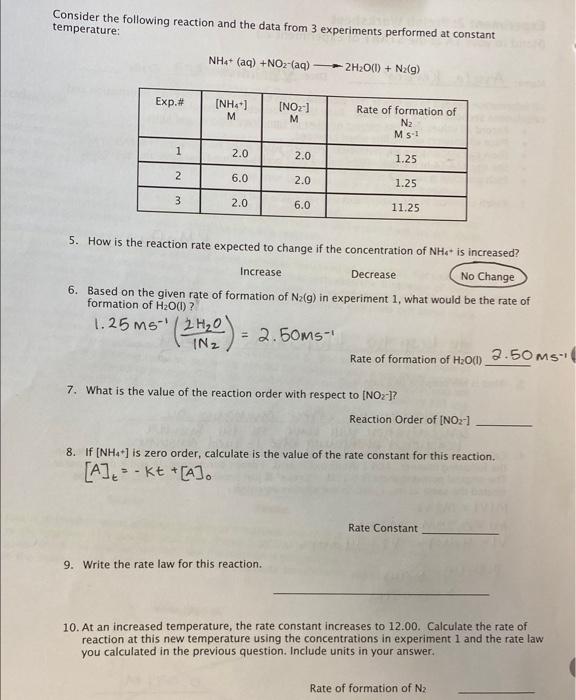
Consider the following reaction and the data from 3 experiments performed at constant temperature: NH4+(aq)+NO2(aq)2H2O(l)+N2(g) 5. How is the reaction rate expected to change if the concentration of NH4 - is increased? Increase Decrease No Change 6. Based on the given rate of formation of N2(g) in experiment 1 , what would be the rate of formation of H2O(1) ? 1.25MS1(1N22H2O)=2.50Ms1 RateofformationofH2O(l)2.5ms. 7. What is the value of the reaction order with respect to [NOz] ? Reaction Order of [NO2] 8. If [NH4+]is zero order, calculate is the value of the rate constant for this reaction. [A]t=Kt+[A]0 Rate Constant 9. Write the rate law for this reaction. 10. At an increased temperature, the rate constant increases to 12.00. Calculate the rate of reaction at this new temperature using the concentrations in experiment 1 and the rate law you calculated in the previous question. Include units in your 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


